Contents
InterLab Study
Since iGEM is an international competition with many teams from all over the world, it is inevitably coping with the problem of unreproducible scientific measurement results. Multiple teams are measuring fluorescence differently, in different units and are evaluating the results non-identically. Therefore, the InterLab Study aims to develop a standardized protocol for repeatable fluorescence measurements of GFP.
Previous studies showed variability in measurements can be reduced by measuring the GFP expression in absolute fluorescence units calibrated against known fluorescent molecule concentrations. Measurements of cell populations still show high variability caused by the number of cells in the sample. Thus, it is necessary to determine the mean expression level of GFP per cell to eliminate a source of variability. This year’s question to be answered is: “Can we reduce lab-to-lab variability in fluorescence measurements by normalizing to absolute cell count or colony-forming units (CFUs) instead of OD?”
Our team followed the standardized procedure guiding us through transformation, inoculation and measurement process. For more information: Fifth International InterLab Measurement Study Following below you can find our results.
Calibration
OD600 Calibration
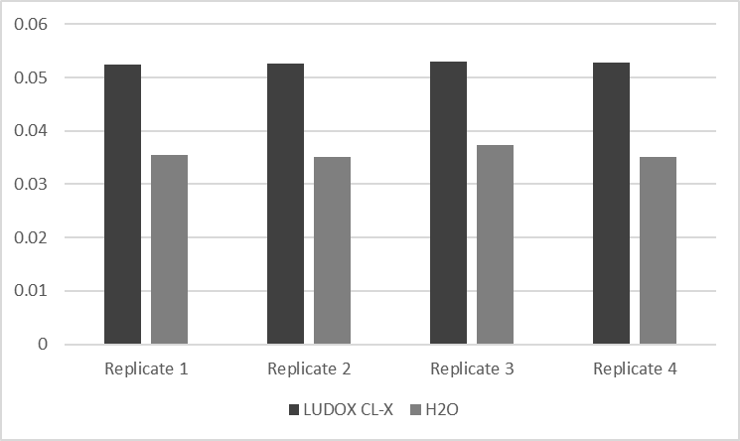
Several measurements of LUDOX-S40 and H2O were spectrometric analyzed below.
| LUDOX CL-X | H2O | |
|---|---|---|
| Replicate 1 | 0.0525 | 0.0355 |
| Replicate 2 | 0.0526 | 0.0351 |
| Replicate 3 | 0.0529 | 0.0373 |
| Replicate 4 | 0.0528 | 0.0352 |
| Arithmetic Mean | 0.0528 | 0.0358 |
| Corrected Absorbance 600 | 0.0169 | |
| Reference OD600 | 0.0630 | |
| Correction Factor | 3.7223 |
Table 1. Absorbance measurement of LUDOX-S40 and H2O of four different replicates.
Defining the correction factor for upcoming experiments allows the conversion of Absorbance 600 from a plate reader into OD600.
Figure 1. Data exported from table 1 in beam chart.
Particle Standard Curve
The absorbance of a dilution series, monodisperse silica microspheres in four replicates, were measured in standard modes in our tecan plate reader (infinite 200Pro).
![]() Table 2. Absorbance 600 measurement of monodisperse silica microspheres in eleven dilutions and one control containing PBS.
Table 2. Absorbance 600 measurement of monodisperse silica microspheres in eleven dilutions and one control containing PBS.
Due to the similarity in size and shape of the microspheres to cells and that there is a known number of particles per volume, the Absorbance 600 can be converted to an estimated number of cells for later experiments. Generated standard curve of absorbance for different numbers of particles can be found below.
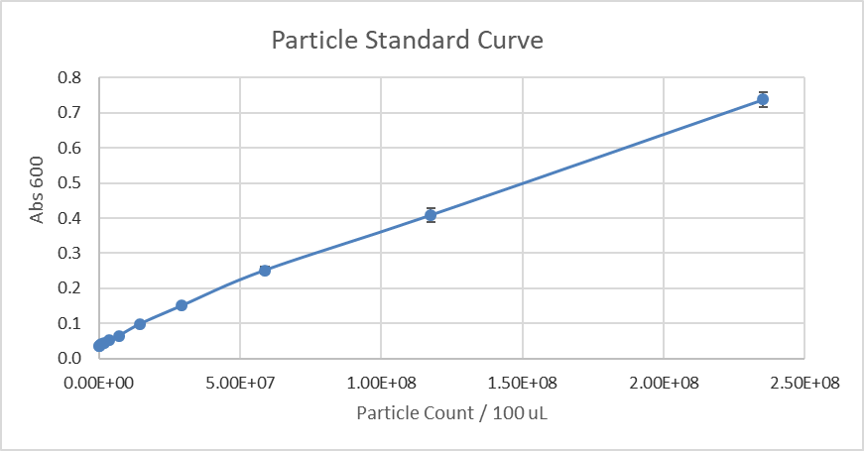 Figure 2. Calibration curve of monodisperse silica microspheres: absorbance measurement of different numbers of particles.
Figure 2. Calibration curve of monodisperse silica microspheres: absorbance measurement of different numbers of particles.
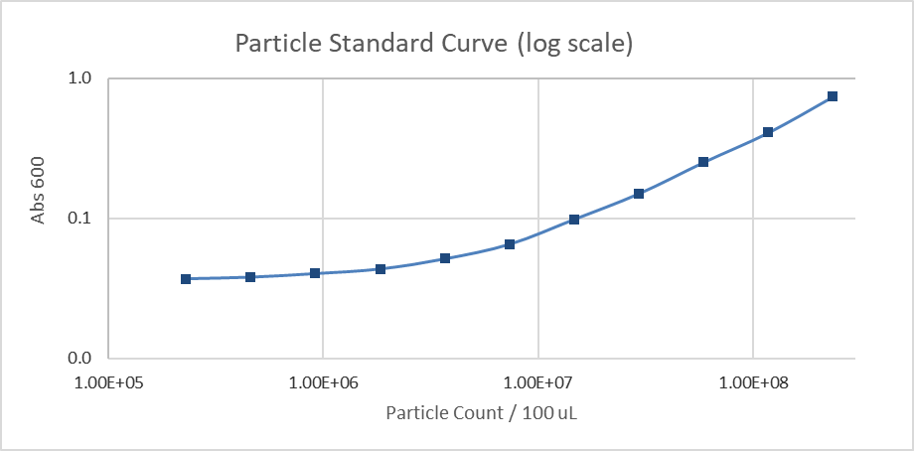 Figure 3. Calibration curve of monodisperse silica microspheres (log scale): absorbance measurement of different numbers of particles.
Figure 3. Calibration curve of monodisperse silica microspheres (log scale): absorbance measurement of different numbers of particles.
Fluorescein Standard Curve
The fluorescence of a dilution series, fluorescein in four replicates, were measured in standard modes in our tecan plate reader.
![]() Table 3. Fluorescence measurement of fluorescein in eleven dilutions and one control containing PBS. Measurement settings for the plate reader: Excitation 485 nm, Emission 530 nm, 50 gains and 20 flashes.
Table 3. Fluorescence measurement of fluorescein in eleven dilutions and one control containing PBS. Measurement settings for the plate reader: Excitation 485 nm, Emission 530 nm, 50 gains and 20 flashes.
Generated standard curve of fluorescence for different fluorescein concentrations can be found below.
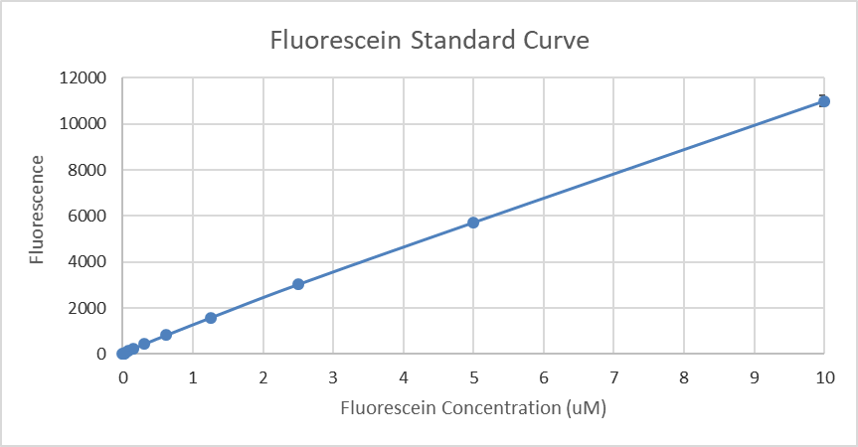 Figure 4. Calibration curve of fluorescein: fluorescence measurement of different concentrations (µM).
Figure 4. Calibration curve of fluorescein: fluorescence measurement of different concentrations (µM).
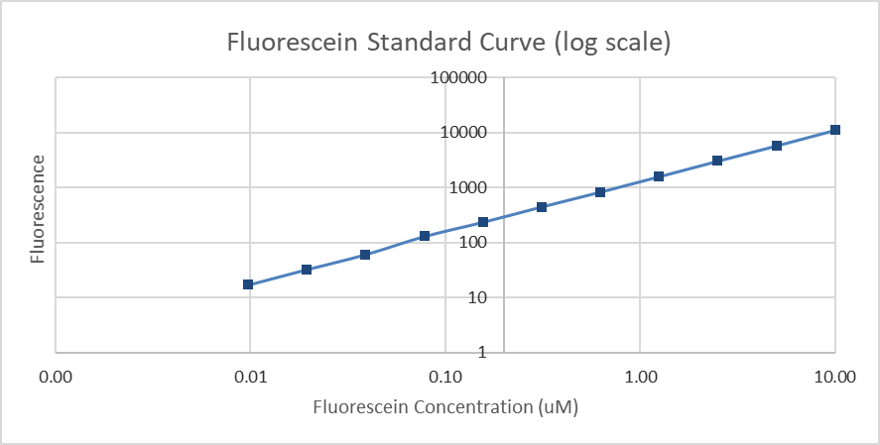 Figure 5. Calibration curve of fluorescein (log scale): fluorescence measurement of different concentrations (µM).
Figure 5. Calibration curve of fluorescein (log scale): fluorescence measurement of different concentrations (µM).
Cell Measurement
Fluorescence and absorbance were measured from six different devices, positive and negative control which are built in pSB1C3 containing chloramphenicol resistance and transformed in E. coli K-12 DH5-alpha. Samples were taken after 0 and 6 hours. The raw data can be found below.
µM Fluorescein per OD after 0 and 6 hours
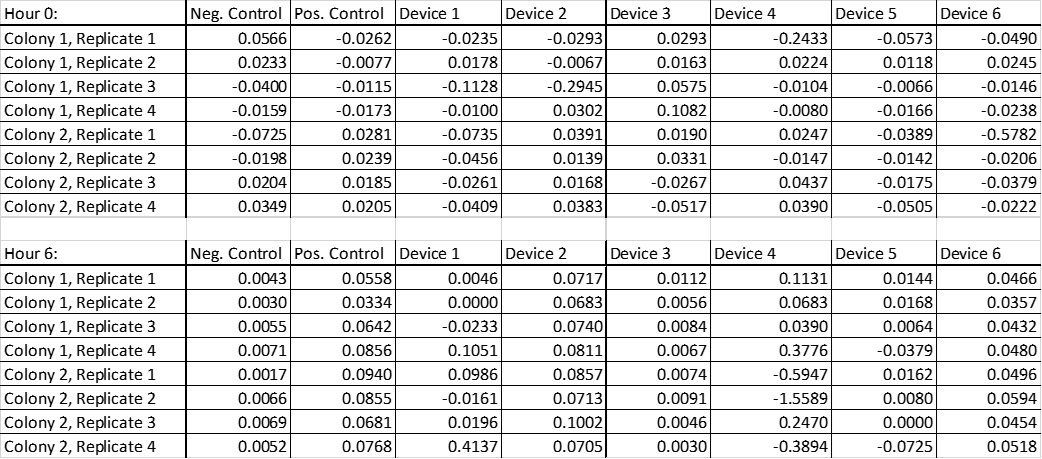 Table 4. Raw data of µM fluorescein per OD from both colonies transformed with negative, positive control, device 1 to 6 in replicates 1 to 4.
Table 4. Raw data of µM fluorescein per OD from both colonies transformed with negative, positive control, device 1 to 6 in replicates 1 to 4.
Molecules of Equivalent Fluorescence Level (MEFL) per particle
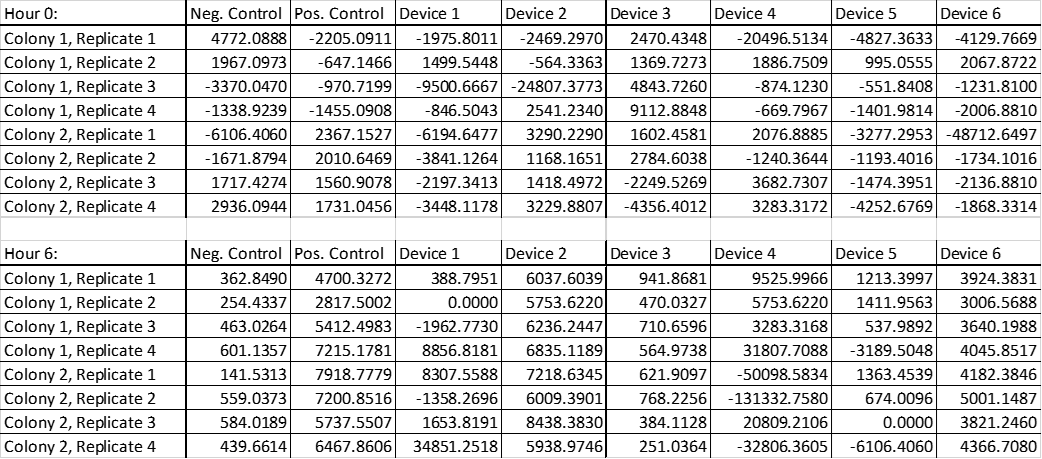 Table 5. Raw data of MEFL (Molecules of Equivalent Fluorescence Level) per OD from both colonies transformed with negative, positive control, device 1 to 6 in replicates 1 to 4.
Table 5. Raw data of MEFL (Molecules of Equivalent Fluorescence Level) per OD from both colonies transformed with negative, positive control, device 1 to 6 in replicates 1 to 4.
In figure 6 you can see, the positive control showed similar data to cells transformed with devices 1 and 2 transformed cells and had the highest fluorescence per OD. Device 4 and 5 showed negative results, but differed from the negative control which still has positive data.
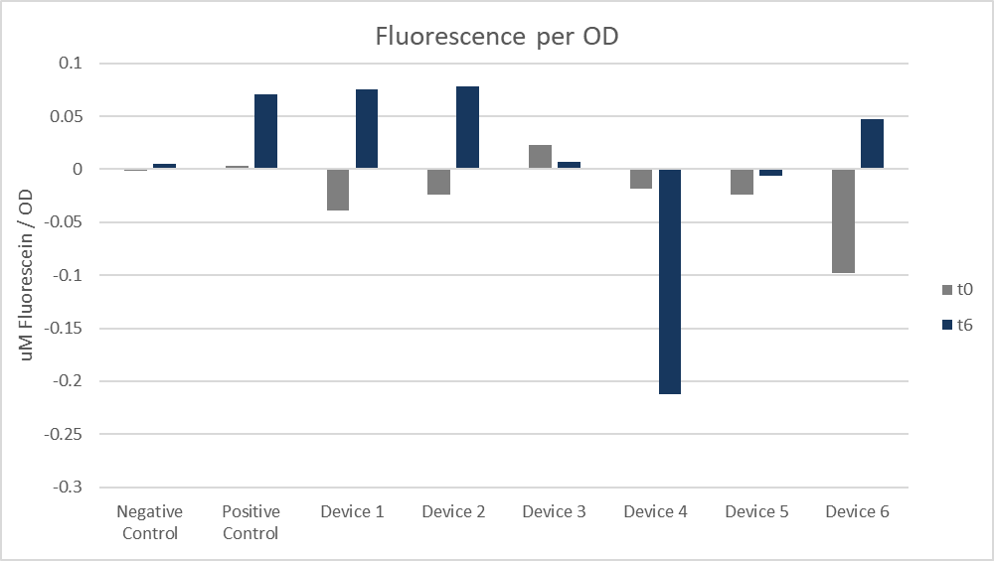 Figure 6. Fluorescence per OD. Arithmetic mean of both colonies and replicates 1 to 4 at 0 hours and 6 hours.
Figure 6. Fluorescence per OD. Arithmetic mean of both colonies and replicates 1 to 4 at 0 hours and 6 hours.
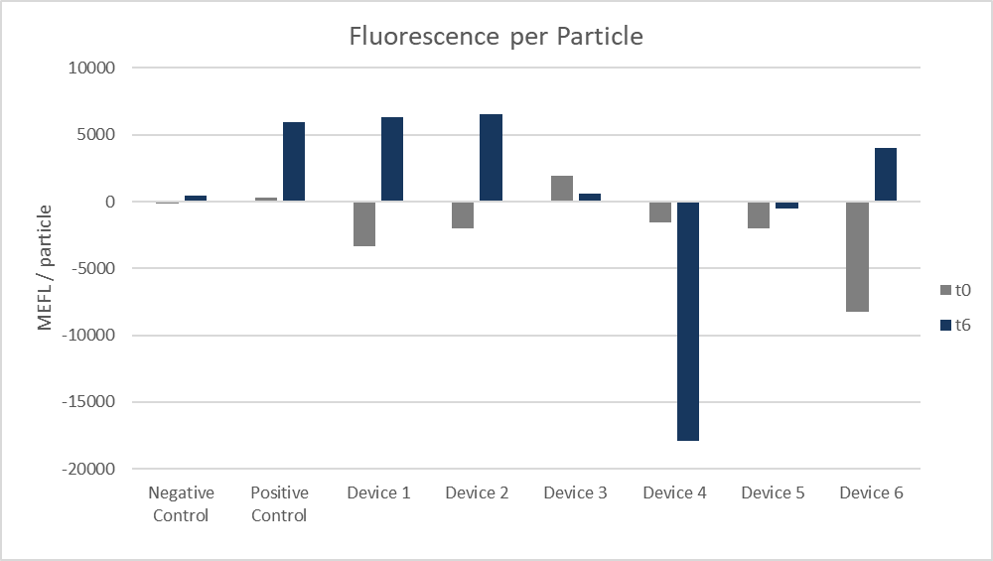 Figure 7. Fluorescence per particle. Arithmetic mean of both colonies and replicates 1 to 4 at 0 hours and 6 hours.
Figure 7. Fluorescence per particle. Arithmetic mean of both colonies and replicates 1 to 4 at 0 hours and 6 hours.