JonathanFu (Talk | contribs) |
JonathanFu (Talk | contribs) |
||
| Line 35: | Line 35: | ||
=== Dynamic light scattering and photocorrelation spectroscopy === | === Dynamic light scattering and photocorrelation spectroscopy === | ||
| − | |||
| − | + | ==== Introduction: ==== | |
Dynamic light scattering is a commonly used method in physics to detemine the size distribution of particles and polymers in a solution. By scattering light from small particles, their geometrical structure and their state of motion can be measured. Dynamic light scattering (DLS) is used to analyse particles undergoing Brownian motion in photon correlation spectroscopy to get insights about the particle size. | Dynamic light scattering is a commonly used method in physics to detemine the size distribution of particles and polymers in a solution. By scattering light from small particles, their geometrical structure and their state of motion can be measured. Dynamic light scattering (DLS) is used to analyse particles undergoing Brownian motion in photon correlation spectroscopy to get insights about the particle size. | ||
| Line 45: | Line 44: | ||
EQUATION HERE!!!! | EQUATION HERE!!!! | ||
| − | + | ==== Measurement: ==== | |
The photon correlation spectroscopy interprets the dynamic light scattering of the suspended particles while taking the size depended Brownian motion into consideration. Small, fast moving particles give different patterns during the spectroscopy than bigger, slow moving particles. This way it is possible to get a precise statement about the particle size distribution. | The photon correlation spectroscopy interprets the dynamic light scattering of the suspended particles while taking the size depended Brownian motion into consideration. Small, fast moving particles give different patterns during the spectroscopy than bigger, slow moving particles. This way it is possible to get a precise statement about the particle size distribution. | ||
Revision as of 19:17, 13 October 2018
Contents
Analysis
NMR-Spectroscopy
Introduction:
NMR-Spectroscopy (nuclear magnetic resonance) is a widely used and versatile analysis to predict the chemical structure of a sample. The method works by placing a sample in a strong magnetic field and exposed to radio waves with different frequencies. Some atomic nuclei will start to resonate as they interact with the radio waves of a certain frequency. The frequency at which the nuclei resonate in respect to the radio waves is determined by the chemical shift of the atom. The chemical shift describes the amount of shielding a nucleus experiences from its surrounding electrons. Nuclei that have a low electron density have a strong chemical shift and therefore resonate at higher frequencies. Thus they appear on the left hand side of the spectrum. The opposite is true for atoms with a weak chemical shift. If groups in a chemical substance have identical surroundings, they appear as a single signal in the spectrum. The integral of the signal tells us how many identical groups correspond to the signal.
Figure 1 1H NMR Spectrum of an organic substance for the illustration of chemical shifts. Notice how there are only 3 signals in the spectrum although there are 8 Hydrogen atoms in the compound because they are chemically identical.
1H-NMR and coupling:
In 1H-NMR-Spectroscopy chemists are looking at the resonance of 1H protons, which is the most common isotope of Hydrogen. Resonating hydrogen atoms can interfere with each other, which leads to a splitting of the signal in the spectrum. This special interference is referred to as coupling and gives even greater insight to the chemical structure of a sample.
Figure 2 illustration of the coupling 1H resonance in a 1H-NMR spectrum. This is giving even further insight into the chemical structure of the component
Gel permeation chromatography
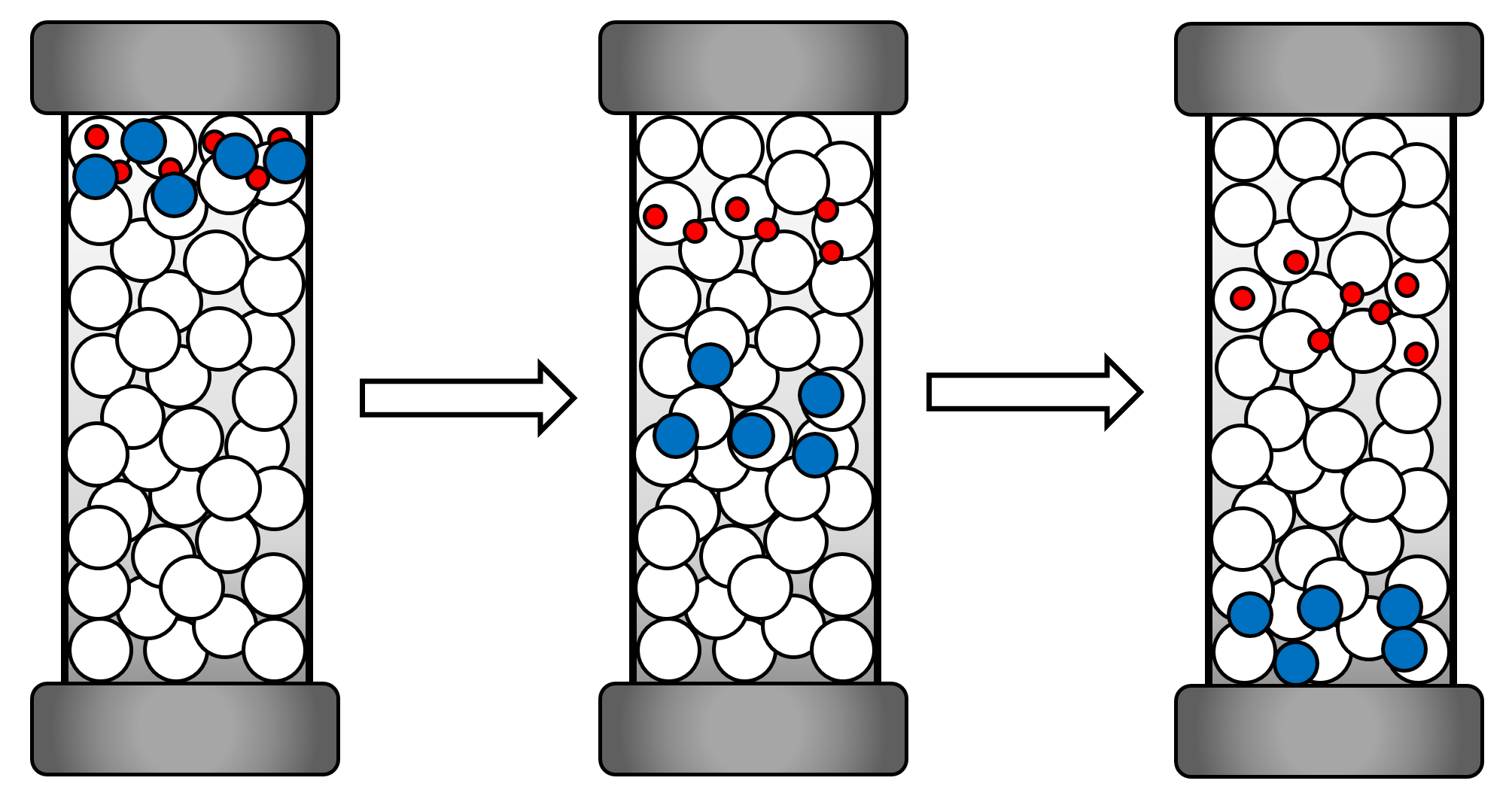
To determine the length of the polymer chains, the gel permeation chromatographic (GPC) turned out to be the method of choice. A pump pulls a sample of the polymer, solved in an eluent through a column, filled with a packing material of various pore sizes. The velocity of the polymer is depending on its size, bigger polymers flow faster than smaller polymers. At the end of the column is a detector (DLS or UV/Vis) measuring the amount of substance coming out of the column in dependence of time. The results are then compared to standard samples and give the information of the molecular weight and through that the length of the polymers.
Figure 3 Illustration of a column inside a GPC. As seen, the bigger polymers move faster than the smaller poylmers on their way through the packing material.
Dynamic light scattering and photocorrelation spectroscopy
Introduction:
Dynamic light scattering is a commonly used method in physics to detemine the size distribution of particles and polymers in a solution. By scattering light from small particles, their geometrical structure and their state of motion can be measured. Dynamic light scattering (DLS) is used to analyse particles undergoing Brownian motion in photon correlation spectroscopy to get insights about the particle size. Correlation of particle size and Brownian motion The Brownian motion of particles is strongly correlated to the size of particles. Understanding this correlation is crutial for the understanding of light scattering spectroscopy. The reason for Brownian motion is the thermal motion of solvent molecules, which transfer their momentum onto the suspended particles when they are colliding. Those momenta do not always cancel out, which results in a net movement of the suspended particle. The particle flow is described by Fick’s first law of diffusion, which also considers concentration gradients. We did our measurements under equilibrated conditions and assume that there are no concentration gradients present. For this reason we are only concerned with the diffusion coefficient which is the proportionality factor of Fick’s first law (Equation 1).
EQUATION HERE!!!!
Measurement:
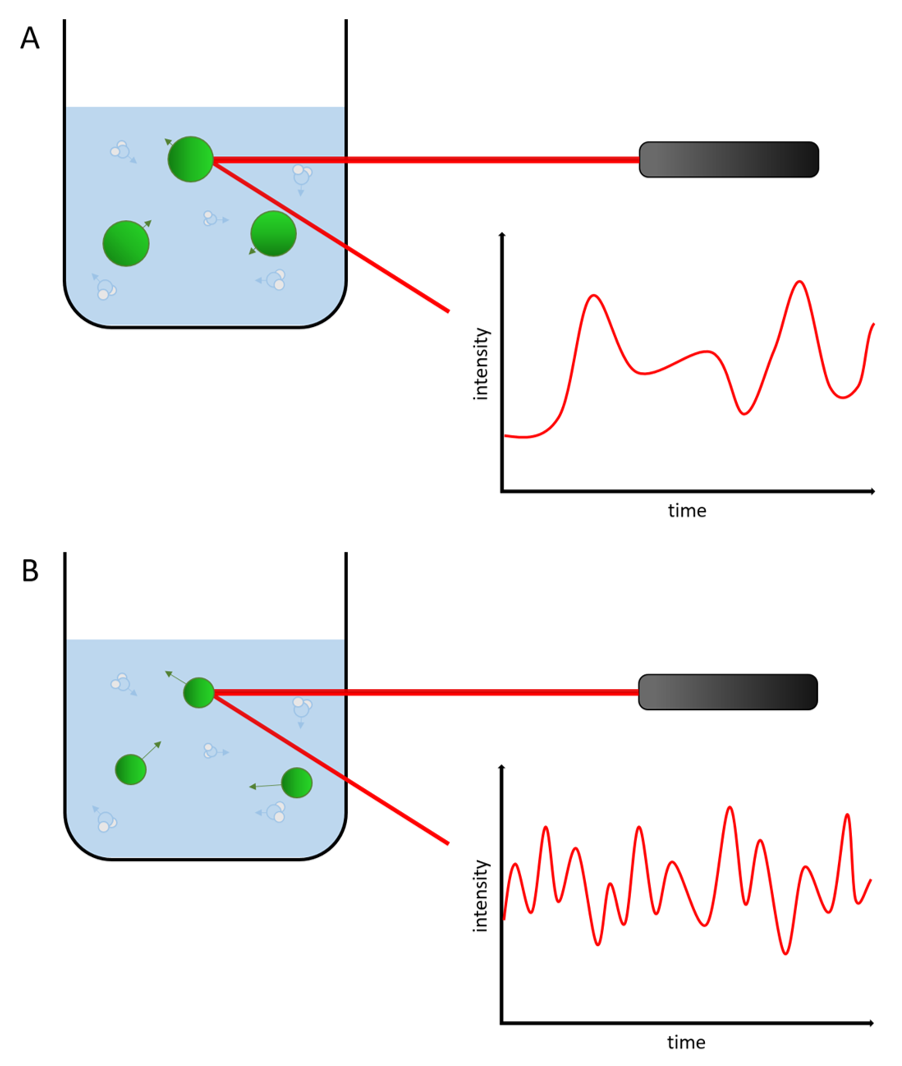
The photon correlation spectroscopy interprets the dynamic light scattering of the suspended particles while taking the size depended Brownian motion into consideration. Small, fast moving particles give different patterns during the spectroscopy than bigger, slow moving particles. This way it is possible to get a precise statement about the particle size distribution.
Figure 4 illustration of the difference in dynamic light scattering with smaller and bigger particles.
Confocal microscopy
Confocal microscopy theory
Confocal microscopy is an imaging technique where only a single plane of the sample is illuminated, therefore just one layer and not the whole sample is visible. The light scans horizontally over the sample to give a 2D image of the sample. The light irradiates on a pinhole in front of the detector, which minimizes out of focus signals resulting in a high contrast picture. The following figure directly compares a confocal microscopy image with a traditional light microscope, where the complete sample is illuminated at a time.
Transmission electron spectroscopy
Transmission electron microscopy (TEM) is a microscopy method in which a beam of electrons is transmitted through a sample to create a picture. Transmission electron microscopes are capable of much higher resolutions, because the de Broglie wavelengths of electrons are much small than the wavelengths of light which are used in light microscopy. The electrons that form the electron ray are emitted from a cathode and accelerated towards the anode which lies under the sample. The electron ray is focused by electron lenses which may operate electrostatically or magnetically. In order to create a picture, the electrons have to pass through the sample and hit the detector. In order to minimize interference of the electrons before they either interact with the sample or the detector, a strong vacuum is applied on the system. This minimizes the probability of the electron ray to come in contact with air molecules. The sample is also very thin, so the electrons actually pass through the sample and only low amounts of electrons are absorbed.