Contents
Applications
Applications of PLA and its co-polymers PLGA and PLGC
PLA is through its easy degradability a good polymer for wrapping material. After using it, it will degrade into nontoxic particles that can’t pollute the environment with micro plastic and if Animals eat it by accident, it will also do no harm to them, because it’s digestible.
Another application for PLA and its Co-polymers PLGA and PLGC is the production of surgery supplies, which are used in the body and stay there for a limited time, like screws for bone surgery or threads for sealing wounds. Those screws and threads don’t have to be removed, because they will be broken down into nontoxic monomers, which are then again broken down by the body and excreted.
The third application of PLGA and especially PLGC is the usage as a drug carrier system. For that the polymers are turned into Nano spheres loaded with therapeutics. The Nano Spheres are taken orally or injected. In the time they degrading, they release the therapeutics and are broken down into nontoxic monomers, which are than again broken down by the body and excreted.
Nanospheres
Abstract
Theory behind the synthesis
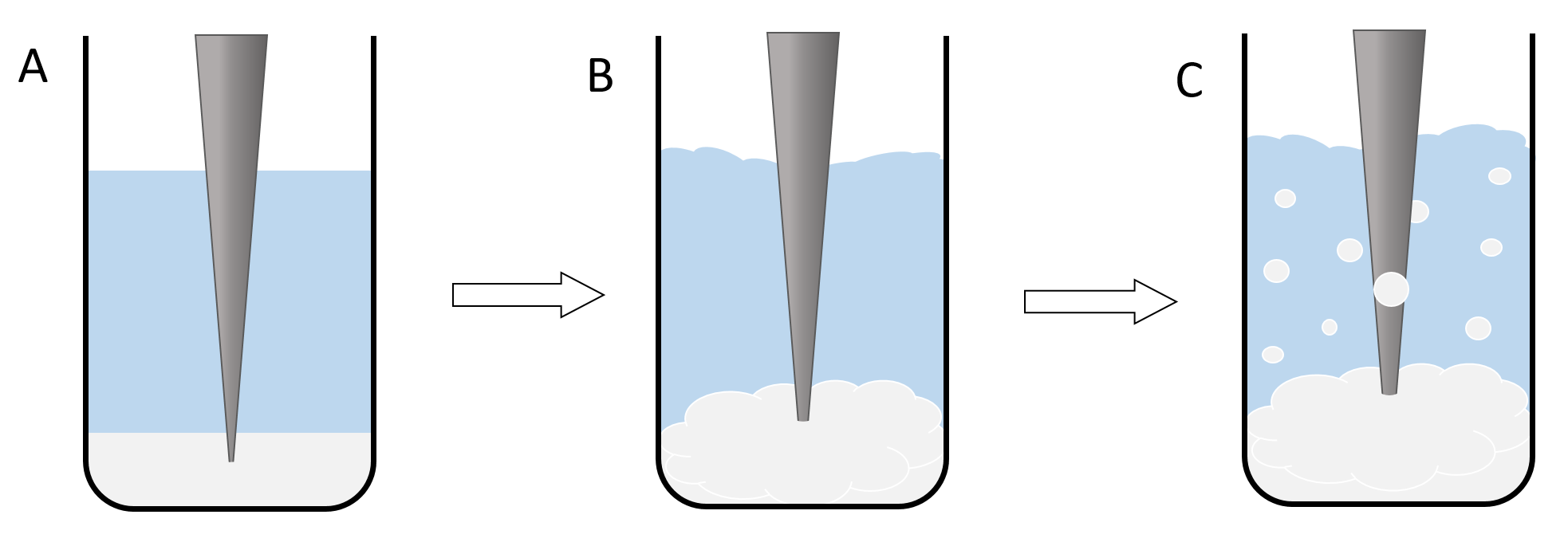
Nanoparticles are synthesized using a bulk polymer. The synthesis does not involve any chemical reactions, only phase transitions and the self assembly process of the polymer to form nanospheres. Two immiscible solutions have to be prepared to generate a system with two phases. The first phase is a solution of the polymer (PLGA or PLGC) in Dichloromethane (DCM). The second phase is a solution of low amounts of an emulsifying agent like Poly-vinyl-alkohol (PVA) in water. The phases first start mixing if the ultrasonic rod is turned on. Due to the presence of the emulsifying agent, little DCM/polymer spheres form in the water phase, which turns the system from a two phase system to an emulsion. The size of the dispersed spheres is dependent on the amount and type of the emulsifying agent and also the intensity of the ultrasonic waves (Figure 1).
Figure 1: illustration of the nanoparticle synthesis. The lowest phase (here white-grey) shows the polymer/DCM Phase and the central phase (here blue) resembles the PVA/water solution. The grey triangle is an illustration of the ultrasonic rod. A shows the basic setup of the reaction, where the two phases are still completely separated and the ultrasonic rod is still inactive. B shows the second phase of the reaction where the rod is turned on and the two phases begin to mix. C illustrates the formation of an emulsion and therefor the formation of the dichlromethane spheres.
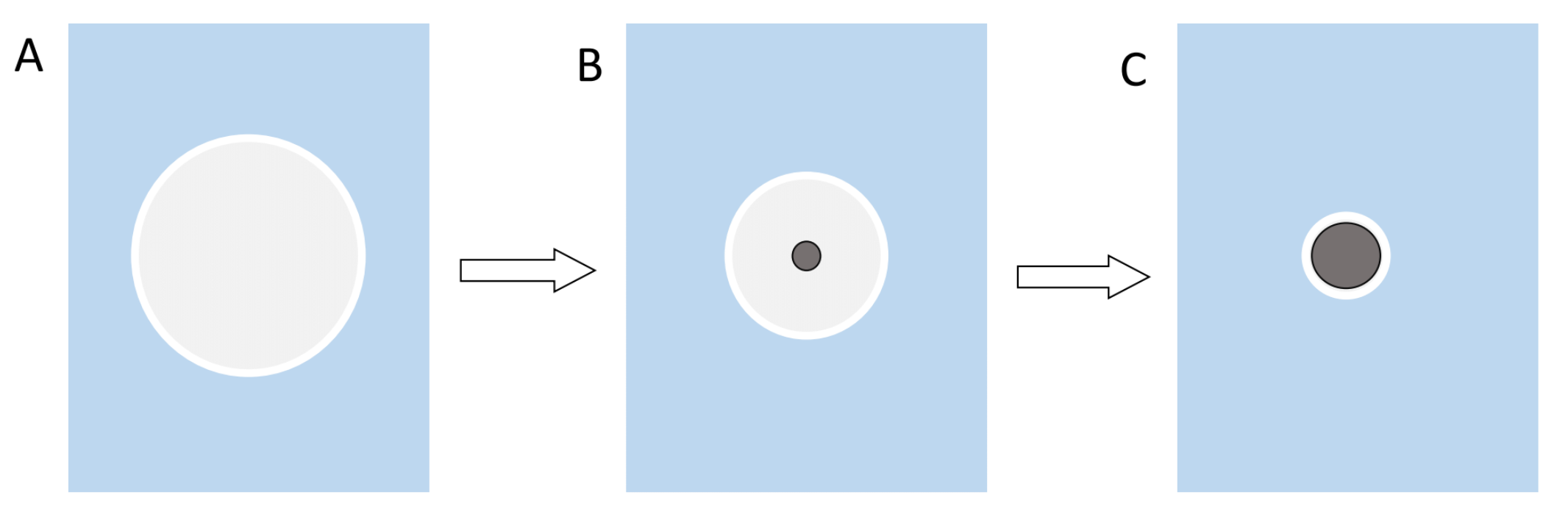
The ultrasonication not only forms an emulsion, but it also creates heat which causes the DCM to evaporate during the synthesis. The evaporation of the solvent then in turn leads to the precipitation and the formation of the particles. The reason for the precipitation is that the solubility of the polymer decreases as the volume of solvent decreases (Figure 2).
Figure 2: Illustration of the precipitation of the polymer and the formation of nanoparticles. A shows the early DCM sphere as an emulsion and a big enough volume for the polymer to be completely in solution. B the volume of the sphere decreases over time until the concentration of the polymer reaches a critical value and it is forced to precipitate out of the solution. C this process continues until the particle reaches its final size.
structure of the particles
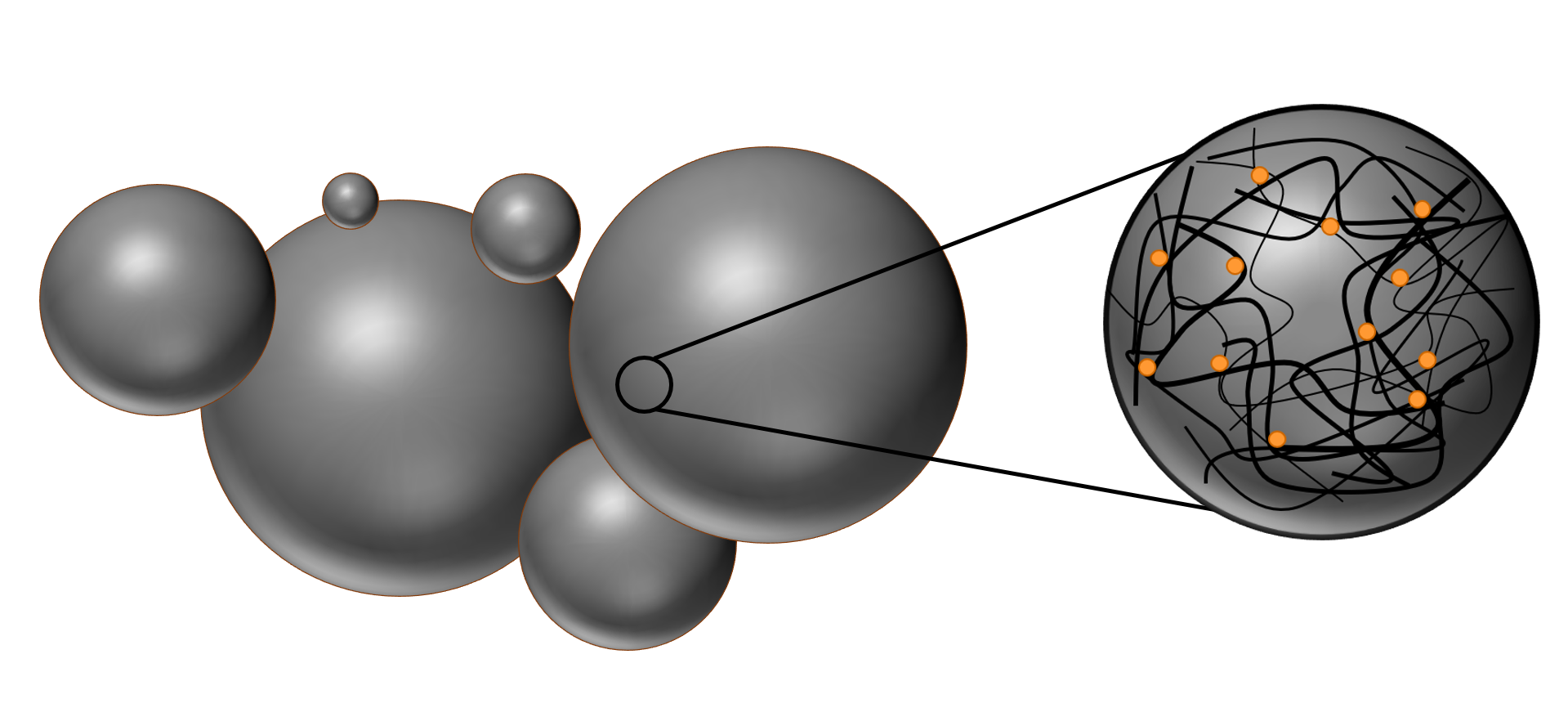
The nanospheres are randomly oriented coils of polymer strings which are held together by intramolecular forces, like Van-der-Waals-forces and dipole-dipole-interactions. The polymer matrix greatly influences the properties of the particles. Additionally, the porous structure eases the diffusion of water inside the matrix and this in turn speeds up the hydrolyzation process of the chemical bonds [link to PLGA background]. Both hydrolyzation and porous structure are especially important for the application in drug delivery [link to applications page].
Encapsulation of molecules
The polymer matrix can enclose molecules like fluorescent dyes or therapeutics like drugs or even proteins. This is exemplary done by simply dissolving the dye molecules together with the polymer in the organic solvent. The synthesis is analogous to the regular nanosphere synthesis, but with a difference in the self-assembly process. Now, not only the polymer precipitates out of the solvent, but the molecules of the dye also stick to the polymer due to hydrophobic interactions between the polymer and the dye.
Figure 3:Illustration of the porous polymer matrix, which is loaded with dye molecules