Contents
Applications
Applications of PLA and its co-polymers PLGA and PLGC
PLA, through its good degradability, is a well-suited polymer for wrapping material. After usage, it will degrade into non-toxic particles that can not pollute the environment with micro plastic. If animals eat it by accident, it will also do no harm to them, because it is digestible.
Another application for PLA and its co-polymers PLGA and PLGC is the production of surgical supplies, which are used in the body and stay there for a limited time, like screws for bone surgery or threads for sealing wounds. Those screws and threads do not have to be removed, because they will be processed into non-toxic monomers by the body and excreted.
We focused on the third application of PLGA and especially PLGC: the usage as drug carrier systems. For that, the polymers are turned into nanospheres loaded with therapeutics. These nanospheres could be taken orally or injected. During degradation, they release the therapeutics and are broken down into non-toxic monomers, which are then dissolved and excreted by the body, causing no further harm.
Nanospheres
Abstract
The usage of nanospheres in medicine continues to gain attention due to its advantages in the function as a drug carrier. The spheres can be loaded with different agents, such as fluorescence markers or drugs e.g. chemo therapeutics. Once in the human body, the spheres slowly degrade and release a calculated amount of drug over time. Therefore, fewer injections per treatment are needed, which is more convenient for patients compared to conventional methods.
Not all polymers are suitable for such applications. It is vital, that the polymer for the production of nanospheres has a specific set of features. First, it needs to be biodegradable in the intended environment (e.g. the human body), and second, both the polymer itself and its monomers have to be naturally non-toxic. PLGA and PLGC break down into the monomers lactic acid, glycolic acid and caprolactones. Both lactid acid and glycolid acid are endogenous in the human metabolism, and caprolactones are harmless for humans in small doses. Therefore, the human body can consume or excrete the monomers easily, when the spheres degrade slowly.
Moreover, PLGA is approved by the Food and Drug Administration (FDA), generally recognized as safe (GRAS) and different products of PLGA for clinical usage are already available on the market. Additionally, the polymers poly-ε-caprolactone (PLC), poly-lactic acid (PLA) and poly-glycolic acid (PGA) were approved by the FDA as well. [1]. Based on these tested polymers, we assume that PLGC will be confirmed by the FDA too in future.
We included the synthesis of nanospheres into our project to show that our polymers have great potential and can even be used for such special and challenging applications as drug-delivery systems [2].
Synthesis background
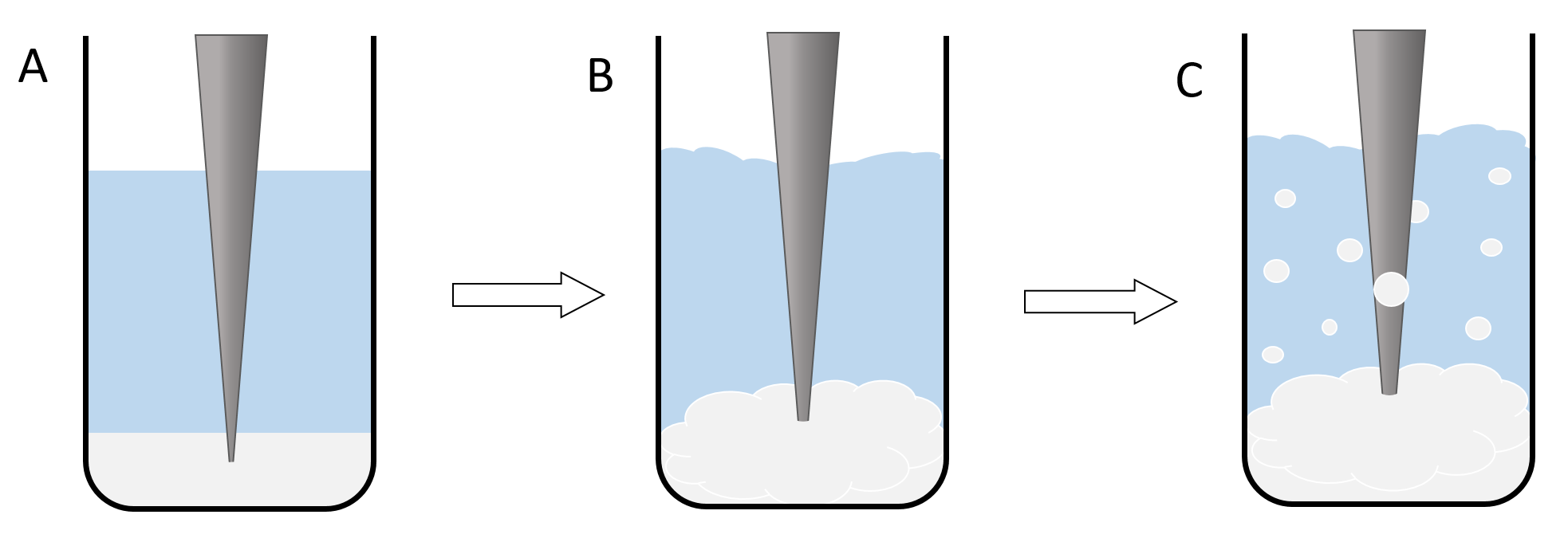
Nanoparticles are synthesized using a bulk polymer. The synthesis does not involve any chemical reactions, only phase transitions and the self assembly process of the polymer to form nanospheres. Two immiscible solutions have to be prepared to generate a system with two phases. The first phase is a solution of the polymer (PLGA or PLGC) in Dichloromethane (DCM). The second phase is a solution of low amounts of an emulsifying agent like Poly-vinyl-alkohol (PVA) in water. The phases first start mixing if the ultrasonic rod is turned on. Due to the presence of the emulsifying agent, little DCM/polymer spheres form in the water phase, which turns the system from a two phase system to an emulsion. The size of the dispersed spheres is dependent on the amount and type of the emulsifying agent and also the intensity of the ultrasonic waves (Figure 1).[3]
Figure 1: illustration of the nanoparticle synthesis. The lowest phase (here white-grey) shows the polymer/DCM Phase and the central phase (here blue) resembles the PVA/water solution. The grey triangle is an illustration of the ultrasonic rod. A shows the basic setup of the reaction, where the two phases are still completely separated and the ultrasonic rod is still inactive. B shows the second phase of the reaction where the rod is turned on and the two phases begin to mix. C illustrates the formation of an emulsion and therefor the formation of the dichlromethane spheres.
The ultrasonication not only forms an emulsion, but it also creates heat which causes the DCM to evaporate during the synthesis. The evaporation of the solvent then in turn leads to the precipitation and the formation of the particles. The reason for the precipitation is that the solubility of the polymer decreases as the volume of solvent decreases (Figure 2).[3]- ↑ J.H.Park, B.K.Lee, S.H.Park, M.G.Kim, J.W.Lee, H.Y.Lee, H.B.Lee, J.H.Kim and M.S.Kim.Int J Mol Sci.18(3): 671 (2017). doi:10.3390/ijms18030671 .
- ↑ H.K.Makadia, S.J.Siegel. Polymers 3(3), 1377-1397 (2011).
- ↑ 3.0 3.1 Katja B. Ferenz, Long-circulating poly(ethylene glycol)-coated poly(lactid-coglycolid) microcapsules as potential carriers for intravenously administered drugs, Journal of Microencapsulation, 2013, 1–11, Early Online [Link oder so].