| Line 18: | Line 18: | ||
[[image:T--TU Darmstadt--Capris,Methodenteil.jpg|820px|alt=Text|Capris Methoden]] | [[image:T--TU Darmstadt--Capris,Methodenteil.jpg|820px|alt=Text|Capris Methoden]] | ||
| + | |||
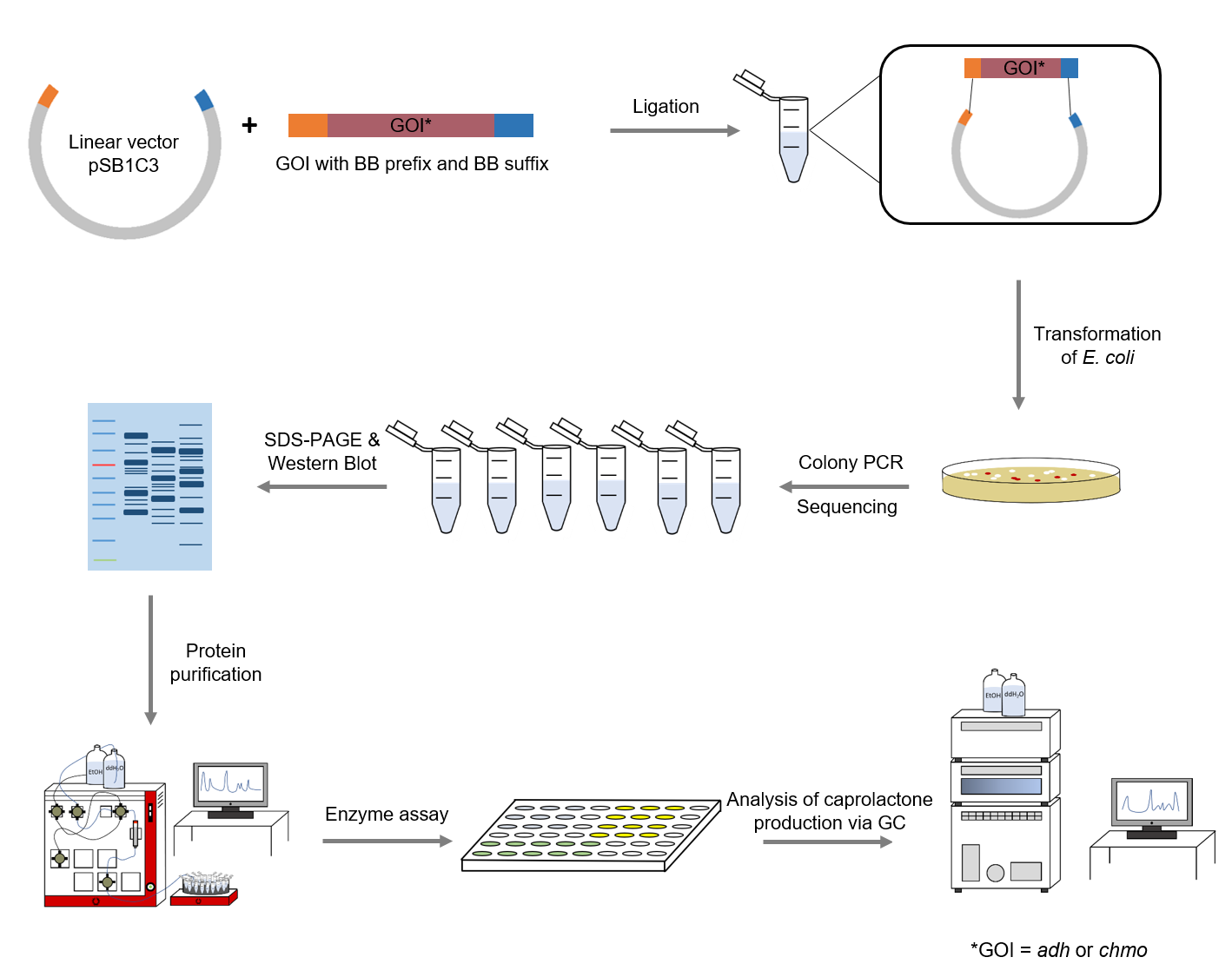
| + | Figure XXX: Schematic representation of work flow. | ||
===Results and Discussion=== | ===Results and Discussion=== | ||
Revision as of 10:04, 13 October 2018
Contents
Caprolactone
Abstract
The chitinase is an enzyme whose ability to break down glycosidic bonds in chitin brings more variability into the molecules. Since not just the grade and pattern of deacetylation, but also the amount of connected chitin monomers influences the entire molecule´s behavior [1], there is a great limitation of the properties and the bioactivity of the products. Its possible implementation in the project shows the future prospects of how chitins and chitosans with all kind of properties can be produced in E. coli.
Introduction
Ɛ-caprolactone is a seven-membered circular lactone. It is used as a building block for several polymers, like in our case poly(lactide-co-glycolide-co-caprolactone) or short PLGC. The Ɛ-caprolactone decreases the degradation time of a polymer by making ester bonds easier accessible for water molecules. [1]Therefore, polymers with caprolactones have a shorter lifetime than polymers without them. Right now, Ɛ-caprolactone is derived from petrochemicals and further chemicals, which are harmful for living beings and their environment. Escherichia coli . In order to produce Ɛ-caprolactone in E. coli, we used the two enzymes, cyclohexanone monooxygenase (CHMO) and alcohol dehydrogenase (ADH).
The most important enzyme for the production of Ɛ-caprolactone in E. coli is cyclohexanone monooxygenase (CHMO). The variant used in this project is derived from Acinetobacter calcoaceticus and has a molecular mass of 60.9 kDa. CHMO is a Baeyer-Villiger monoxygenase, which catalyses the direct conversion from a ketone into an ester. In our case, CHMO catalyzes the conversion from cyclohexanone into Ɛ-caprolactone. The enzyme requires oxygen as well as the cofactor NADPH to catalyze this reaction. Since the wildtype of the enzyme is unstable and therefor consequently unsuitable for large scale application, a double mutant of CHMO has been used in this project (C376L/M400I). <ref= “Hauptpaper Capris”/>These mutations lead to an higher tolerance against oxidation, and consequently lead to an enhanced stability and functionality of CHMO.
The other enzyme we used is alcohol dehydrogenase (ADH) from Lactobacillus kefir. ADH has two functions - first, it converts cyclohexane into cyclohexanone and secondly, it recycles the cofactor NADP+ to NADPH, enabling further CHMO activity. Hence, the combination of these two enzymes makes the process self-sustaining. ADH is NADP+-dependent and has a molecular mass of 26.8 kDa.
In order to achieve a maximal output the enzymes should be used in a 1:10 ratio (ADH:CHMO). ). <ref= “Hauptpaper Capris”/> Sandy Schmidt, Angewandte Chemie, Wiley Online Library, 2015.</ref>
Methods
Figure XXX: Schematic representation of work flow.
Results and Discussion
Outlook
There are several things we did in our project to enhance the activity of the cyclohexanone monooxygenase (CHMO), which is catalyzing the ε-caprolactone-forming step. First of all, we used the double mutant C376L/M400I of CHMO. This mutation leads to a higher tolerance against molecular oxygen as well as an enhanced stability, which leads to a longer period of ε-caprolactone production. Combining CHMO with the alcohol dehydrogenase (ADH) is another way how we want to upscale the production of ε-caprolactone. CHMO is NADPH-dependent and since NADPH is an expensive chemical, its use in industry processes economically unfeasible. The function of ADH is to regenerate the NADPH. By adding the enzyme to the organism, it makes the process self-sustaining. The highest product concentration can be reached when using ADH and CHMO in a ratio of 1:10. ). ). [2] (Graphic Mechanisms) There are a lot more things which can be done to enhance the product yields from CHMO, which we were unfortunately unable to do because of the time limit. One problem of the CHMO is product inhibition. This problem can be faced by additionally adding CAL-A lipase from Candida Antarctica. CAL-A lipase performs an enzymatic ring opening polymerisation of the ε-caprolactone thus creating ε-caprolactone oligomers and can thereby avoid the inhibition. As it can be seen in figure nr. Xx, this leads to a way higher yield. Cyclohexanol is the substrate added to enable the reaction to take place. The effectiveness of CHMO can be improved further by adding bacterial haemoglobin from Vitreoscilla stercoraria (Lee et al. 2014). CHMO does not only require NADPH to catalyze the conversion from cyclohexanone into ε-caprolactone but does also need oxygen in order to work efficiently. The haemoglobin is supplying the enzyme with oxygen which leads to a higher yield.
CHMO has the potential to produce high amounts of ε-caprolactone. Especially when the process gets further optimisied, it is worth considering it as an alternative to the chemical production of ε-caprolactone.