Jaminersss (Talk | contribs) |
|||
| Line 81: | Line 81: | ||
| − | [[image:T--TU_Darmstadt--Enzymassay_GLYR1_Hefen.jpg|450px |alt=Text|Enymeassay GLYR1]] [[image:T-- | + | [[image:T--TU_Darmstadt--Enzymassay_GLYR1_Hefen.jpg|450px |alt=Text|Enymeassay GLYR1]] [[image:T--TU Darmstadt--Calibrationcurve GLYR1 Hefen.png|450px |alt=Text|Enymeassay GLYR1]] |
Revision as of 08:57, 14 October 2018
Contents
Glycolic Acid Production in S. cerevisiae
Abstract
For the production of a biodegradable polymer, our goal was to produce glycolic acid as one of the monomers in Saccharomyces cerevisiae. Glycolic acid production from glyoxylate was initiated by the heterologous expression of glyoxylate reductase 1 (GLYR1). To increase the yield of glycolic acid, we intervened in the glyoxylate cycle by overexpression of the isocitrate lyase 1 (ICL1), deleting the malate synthase 1 (MLS1) and the isocitrate dehydrogenase 2 (IDP2)[1]. We produced AtGLYR1 in S. cerevisiae, used western blots for verification, purified it via Strep-tag affinity chromatography and characterized its activity through an NADPH assay.
Introduction
By modifying the natural glyoxylate cycle, we aim to generate a biosynthetic pathway to produce glycolic acid in S. cerevisiae using the strains CEN.PK-1C and H3847[1]
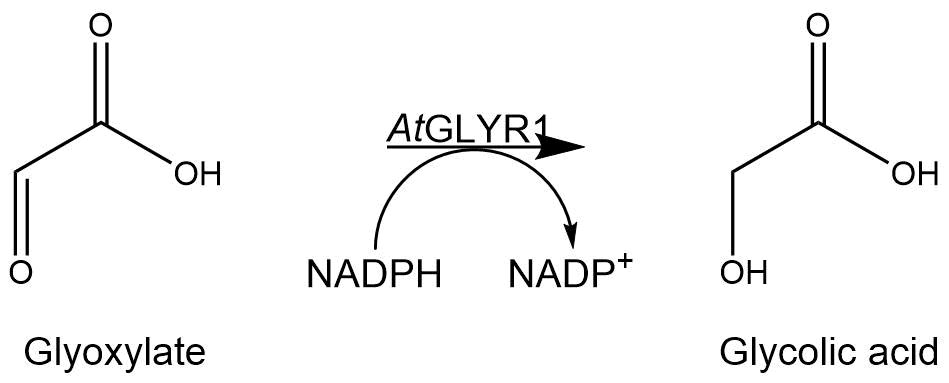
GOR1 is the natural occurring gene in S. cerevisiae, which encodes the enzyme converting glyoxylate into glycolic acid by reducing the aldehyde group in a NADPH-dependent reaction. Since the gene is not expressed[2] under normal growth conditions and the encoded glyoxylate reductase shows moderate affinity to the substrate glyoxylate, we used GLYR1 from Arabidopsis thaliana instead. Previous studies reported an enhanced affinity of AtGLYR1 to glyoxylate[3] and also enhanced activity [1]. The enzyme has a molecular weight of 30,7 kDa [4].
[Bild Glyr1]
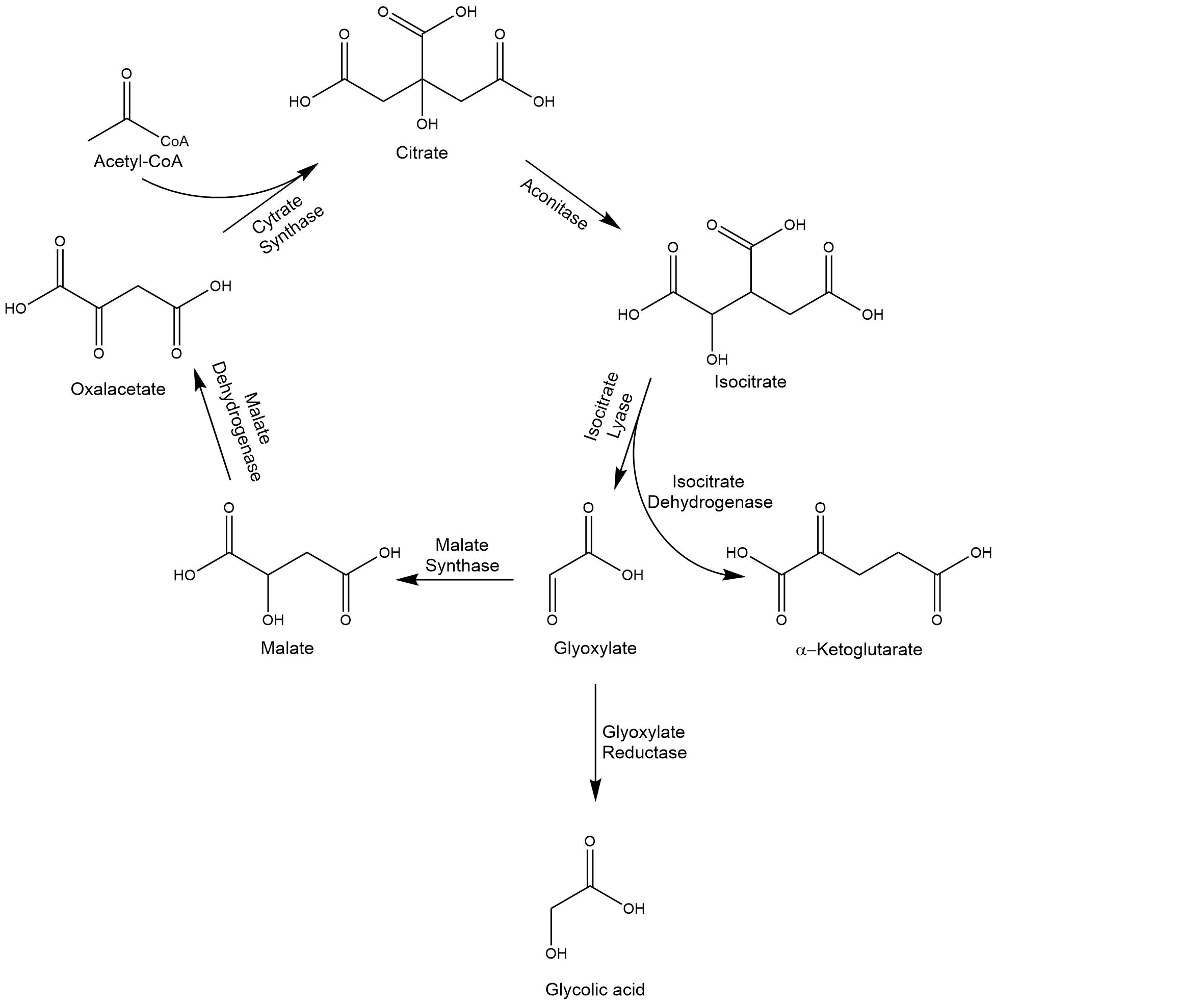
Accumulating glyoxylate benefits the yield of glycolic acid. The isocitrate lyase 1, ICL1, catalyzes the reaction from isocitrate to glyoxylate. In the formation of glyoxylate, succinate occurs as a byproduct of the reaction. By overexpressing ICL1, a high substrate concentration is ensured. ICL1 has a molecular weight of 62.4 kDa[5].
[Bild ICL1]
The accumulation of glyoxylate is supported by the deletion of MLS1 and IDP2, since these enzymes degrade glyoxylate and isocitrate in unwanted side reactions. Consequently, previous deletions of both enzymes in S. cerevisiae significantly increased glycolic acid production titers[1]. MLS1, a malate synthase, catalyzes the conversion from glyoxylate to malate, and IDP2, an isocitrate dehydrogenase, catalyzes the oxidation of isocitrate to α-ketoglutarate.
As a result of our metabolic engineering approach, we were able to prove the activity of the glyoxylate reductase AtGLYR1. Due to the lack of time we were not able to detect the glycolic acid production in S. cerevisiae.
Methods
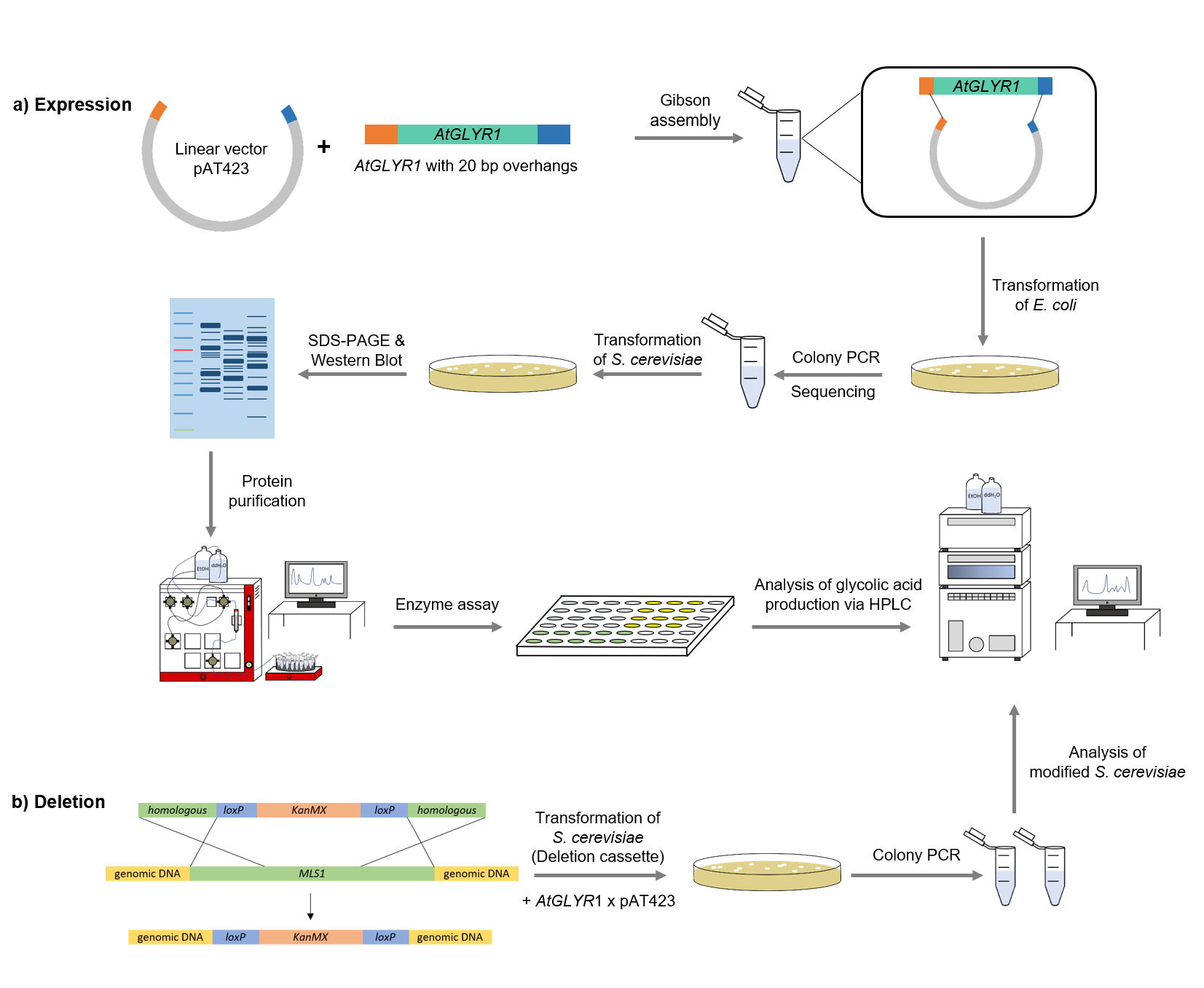
Figure XXX: Schematic representation of work flow.
Cloning
Firstly, the sequence for AtGLYR1 was modified with a Strep-tag on the 3´end and 20 base pairs overhangs at the two ends of the gene, which are complementary with the alcohol dehydrogenase promotor (ADH) of the pAT423 plasmid. The sequence was ordered from Integrated DNA Technologies (IDT) and inserted into the pAT423 plasmid using the Gibson assembly method. E. coli TOP10 were transformed with generated plasmids and positive colonies were identified via colony PCR and DNA sequencing.

 [[->2 Plasmidkarten pro Teamseite]]
[[->2 Plasmidkarten pro Teamseite]]
Deletion
MLS1 and IDP2 were deleted from the genome of the S. cerevisiae using deletion cassettes, which were integrated into the genome by homologous recombination. Both deletion cassettes (KanMX-cassette and Leucin-cassette) were first amplified via PCR. The primers were binding to the loxP-sites and had a 40 bp homologous region to the gene. After that, S. cerevisiae cells were transformed with the linearized amplifed deletion cassettes, with the result that MLS1 was deleted using the KanMX- and IDP2 using the Leucin-cassette.
SDS-PAGE and Western Blot
To verify that AtGLYR1 was expressed and the corresponding protein was translated, a SDS-PAGE was performed, followed by a western blot. The resulting bands were compared to the expected protein size of 32,9 kDa.
Purification
After expression of AtGLYR1 in S. cerevisiae, a GE Healthcare ÄKTA Pure machine was used to purify the desired enzyme via Strep-tag.
Enzyme assays
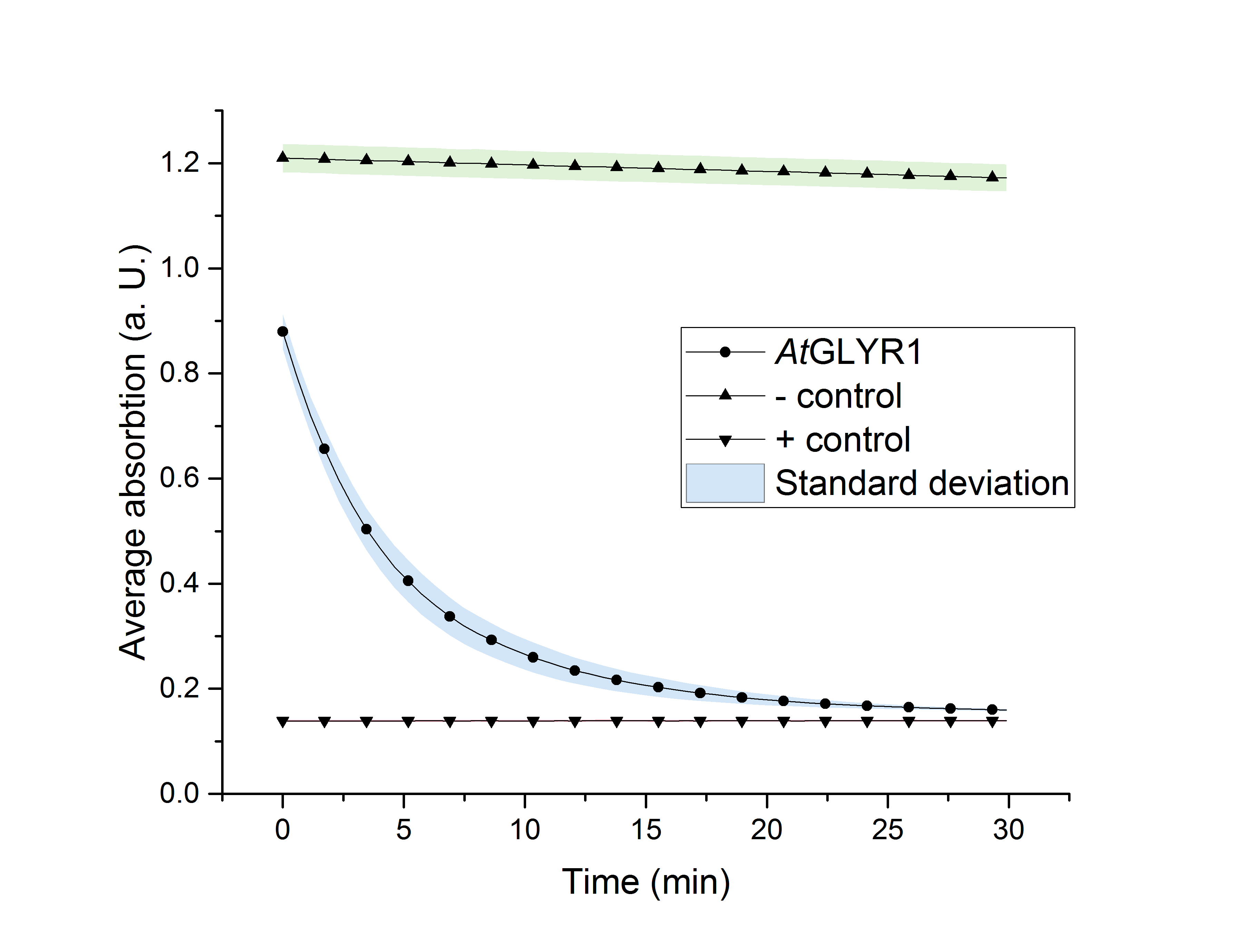
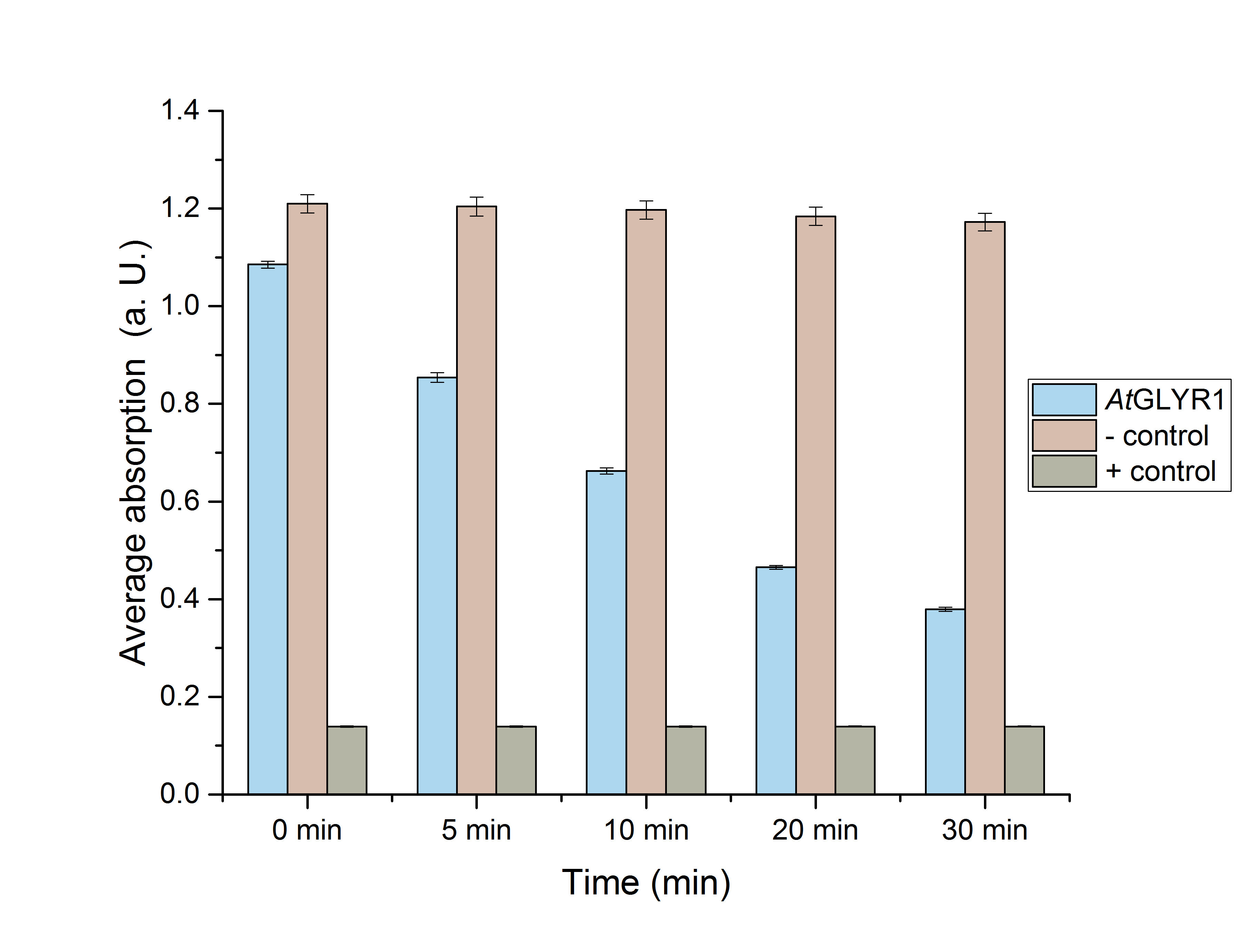
The assay for the glyoxylate reductase AtGLYR1 is based on the different absorption maxima of NADPH (absorption maximum at 340 nm) and NADP (maximum at 260 nm). During the reaction, the enzyme uses NADPH as a cofactor. NADPH is converted into NADP, which leads to a decrease of absorption at 340 nm. By measuring the absorption at a level of 340 nm over time, it is possible to quantify the enzyme activity and infer glycolic acid production.
HPLC analysis
To detect the produced monomer (glycolic acid), as well as the the precursors, high-performance liquid chromatography (HPLC) was utilized. An organic acid separation column as stationary phase and sulfuric acid as mobile phase was used. This allowed the separation of isocitrate, glyoxylate and glycolic acid. Signals were recorded by a refractive index detector.
Results and Discussion
To increase the yield of glycolic acid MLS1 (malate synthase) and IDP2 (isocitrate dehydrogenase) were deleted. MLS1 converts glyoxylate to malate. Therefore, the amount substrate for GLYR1 is minimized. To bypass this problem MLS1 was deleted. IDP2 converts isocitrate to α-ketoglutarate. Isocitrate is also used by ICL1 as a substrate to produce glyoxylate. Glyoxlyate is the precursor of glycolic acid. Therefore, as much isocitrate as possible should be converted to glyoxylate instead of α-ketoglutarate. The deletions were performed by transformation of CEN.PK 1C with a deletion cassette which contained homologous regions to the flanking areas of the gene of interest (for a more detailed explanation see Methods). To determine whether or not the deletion of MLS1 and IDP2 worked, a PCR with two sets of primers were used. If the deletion was not successful only the primer within the gene of interest would bind. If the deletion was successful the primer would bind in the deletion cassette. Therefore, two PCRs per colony were performed. To see if the gene of interest or the deletion cassette was part of the genome, the PCR product was applied to an agarose gel (see Fig. X).
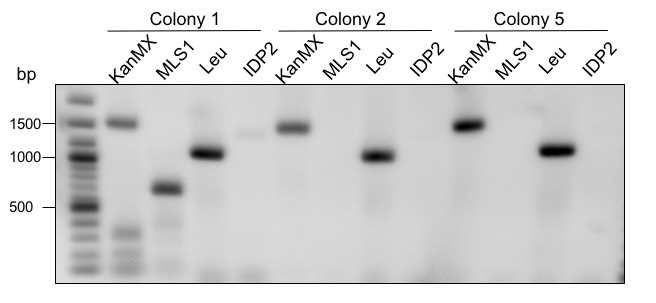
Fig.X Gel electrophoretic separation for control of deletions. KanMX= primers bind in the KanMX cassette, MLS1 = primers bind in the MLS1 sequence. Leu= primers bind in the Leucin cassette, IDP2 = primers bind in the IDP2 sequence. 2-log ladder from NEB was used.
Figure X shows 3 differnet colonies which were screened. Colony 1 shows a fragment for KanMX as well as MLS1. If the deletion had worked only a fragment for KanMX should be visible. Therefore, colony 1 is a mixed culture. The expected fragment sizes are 1650 bp for the KanMX cassette and 1012 bp for MLS1. Colony 2 and 5 only show a fragment for KanMX but not for MLS1. This means that the deletion was performed successful. IDP2 was deleted using a Leucin cassette instead of KanMX. The expected fragment sizes for the cassette and IDP2 are XXX bp and XXX bp. All three tested colonies show a clear fragment for Leucin. The Deletion of IDP2 was successful. Colony 1 was not used for further experimental procedures.
To produce glycolic acid in S. cerevisiae a glyoxylate reductase from Arabidopsis thaliana needs to be expressed. The native glyoxylate reductase GOR1 does not have an affinity as high to glyoxylate as GLYR1 from A. thaliana. A codon optimized version of the sequence coding for AtGLYR1 containing a strep-Tag was ordered at IDT. The sequence was inserted into a shuttle vector (pAT423) which can be used for geneexpression in S. cerevisiae as well as in in E. coli. The cloning was done using the Gibson Assembly method. The accuracy of the cloning was confirmed by sequencing.
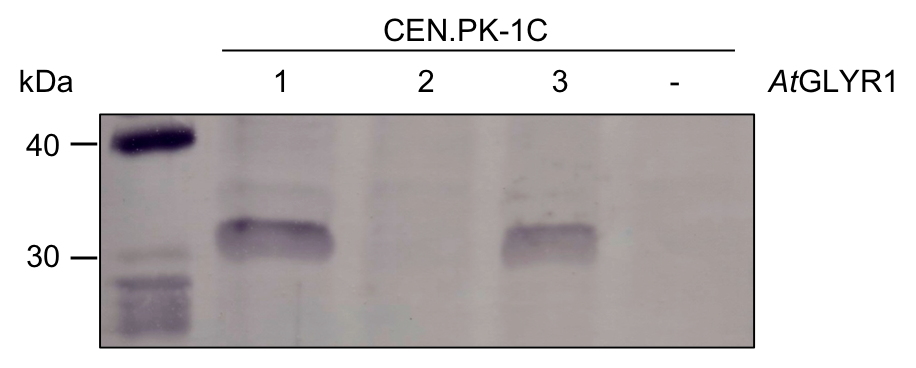
To test if AtGLYR1 is translated in S. cerevisiae we performed western blot analysis. We tested three colonies which were grown on selective medium after transformation with pAT423xGLYR1. As a negative control untransformed CEN.PK 1C was used. The results can be seen in Figure Y. The protein was designed to contain a Strep-tag. Therefore, an anti-Strep antibody was used to detected the tagged GLYR1. The expected protein sizes is 31,9 kDa.
Fig.Y Western blot for AtGLYR1. Three colonies were screened for the translation of AtGLYR1 in S. cerevisiae. + = CEN.PK 1C was transformed using pAT423xAtGLYR1; - = Negative control of untransformed CEN.PK 1C
| glyr1 [µg/ml] | A (t=0 min) | A (t=5 min) | A (t=10 min) | A (t=20 min) | A (t=30 min) | Ν |
|---|---|---|---|---|---|---|
| 8,7 | 0,879±0,023* | 0,433±0,028* | 0,27±0,021* | 0,179±0,007* | 0,16±0,002* | 3 |
| neg. | 1,209±0,019* | 1,204±0,019* | 1,197±0,019* | 1,184±0,018* | 1,172±0,018* | 3 |
| pos. | 0,139±0,001 | 0,139±0,001 | 0,139±0,001 | 0,139±0,001 | 0,14±0,001 | 3 |
Outlook
Due to metabolic engineering of the glyoxylate cycle in S. cerevisiae, we were able to produce glycolic acid as one of our monomers for PLGA- and PGLC-synthesis. It has been shown that yeast acts as a suitable organism for genetic modifications and production of small organic acids. Yet, an emerging number of different yeast species have established themselves as microbial cell factories with at present unknown potential to further increase the yield of metabolic products. Recent work with Kluveromyces lactis (Koivistoinen et al. 2013) suggests that a higher titer of glycolic acid can be received by implementation of similar biosynthetic approaches on different organisms. Obtaining a quantitative analysis of the so produced monomers would therefore be of particular relevance for the comparison of the chosen production strains. In this regard, the currently applied method (Zahoor et al. 2014) including high-performance liquid chromatography with use of a refractive index detector for signal recording can be complemented with external standards in order to create a quantifying calibration curve.
Furthermore, the construction of a BioBrick-compliant shuttle vector for overexpression, protein purification with Strep-tag afinity chromatography and subsequent activity assays, similar to those performed on AceA in E. coli, is the next step in the process of glycolic acid production. For the construction of a stable GLYR1-encoding strain, the glyoxylate reductase gene could be integrated into the genome via homologous recombination, since transformed populations can suffer from plasmid instability (Zhang et al. 1996). Since the reaction catalyzed by GLYR1 is depending on NADPH, the effect of cofactor limitation and regenerating systems, like we used for the production of ε-caprolactone, could also be investigated. Coordinating the two-gene expression ratio of heterologous GLYR1 and overexpressed ICL1 is one potential future outlook.
Source: Koivistoinen, O. M., Kuivanen, J., Barth, D., Turkia, H., Pitkänen, J.-P., Penttilä, M., & Richard, P. (2013). Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis. Microbial Cell Factories, 12, 82. Zhang, Z., Moo-Young, M., & Christi, Y. (1996). Plasmid stability in recombinant Saccharomyces cerevisiae. Biotechnology Advances, 14(4), 401-435. Zahoor, A., Otten, A., & Wendisch, V. F. (2014). Metabolic engineering of Corynebacterium glutamicum for glycolate production. Journal of Biotechnology, 192, 366-375
- ↑ 1.0 1.1 1.2 1.3 Glycolic acid production in the engineered yeasts Saccharomyces cerevisiae and Kluyveromyces lactis [1].
- ↑ The ORF YNL274c (GOR1) codes for glyoxylate reductase in Saccharomyces cerevisiae [2].
- ↑ Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta [3].
- ↑ UniProtKB - Q9LSV0 (GLYR1_ARATH) [4].
- ↑ ICL1 / YER065C Overview [5].