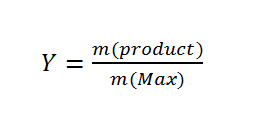| Line 180: | Line 180: | ||
===Outlook=== | ===Outlook=== | ||
| − | PLGA and PLGC were produced using an insufficient magnetic stirring mechanism, which was not able to stir the reaction mixture at higher viscosity up to the end of the reaction. That led to a low conversion, which makes the results hardly reproducible. To avoid that, the reaction vessel needs to be designed in a way, which provides a continuous stirring all time throughout the reaction. The biggest difficulty lies in the achievement being able to have a mechanical stirring mechanism and pulling all humid atmospheric air out of the reaction vessel. Those conditions are not achievable with | + | PLGA and PLGC were produced using an insufficient magnetic stirring mechanism, which was not able to stir the reaction mixture at higher viscosity up to the end of the reaction. That led to a low conversion, which makes the results hardly reproducible. To avoid that, the reaction vessel needs to be designed in a way, which provides a continuous stirring all time throughout the reaction. The biggest difficulty lies in the achievement being able to have a mechanical stirring mechanism and pulling all humid atmospheric air out of the reaction vessel. Those conditions are not achievable with the laboratory equipment, which we can afford. With both conditions given, we would be able to get better reproducible results and more constant chain lengths. And with the polymers produced that way we would be also able to add additives and produce for example composite materials out of them. |
Revision as of 14:38, 15 October 2018
Contents
PLGA
Abstract
In order to produce Poly-lactid-co-glycolide (PLGA), there are many different ways of polymerization. Given that our polymer is a polyester, there are two possibilities of polymerization. The first one is a polycondensation directly out of the monomers lactic- and glycolic acid by eliminating water molecules. A problem of this method is, that the resulting polymer has lower molecular weights, which diminish the mechanical characteristics. Another possibility is to use an anionic ring opening polymerization. However, it is necessary to dimerize the monomers before using them for such a polymerization, otherwise an anionic ring opening polymerization would not work. The reason why we chose an anionic ring opening polymerization for our project is the fact that such a polymerization causes high molecular weights and is controllable by the amount of initiator used in the set up. The manufactured polymers are then analyzed by gel permeation chromatography (GPC) to determine their molecule weight. Polymers with a sufficient enough weight are then used to manufacture nanospheres.
Introduction
Like many other common plastics like Polycarbonate (PC) or Polyethylenterephthalate (PET), PLGA (poly-lactid-co-glycolid) belongs to the family of polyesters. PLGA is build from lactic acid and glycolic acid monomers and is characterized through both a high mechanical resistance and being a thermoplastic.
Synthesis of PLGA
Mechanism - Anionic ring opening polymerization
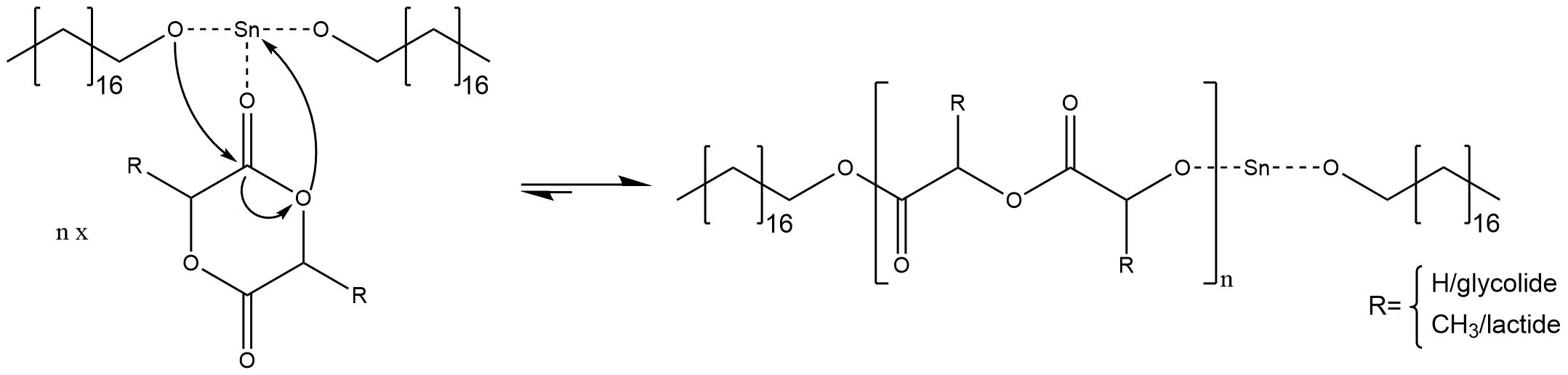
In order that an anionic ring opening polymerization takes place, it needs a nucleophilic initiator, which attacks the carbon atom next to the hetero atom(s). The initiator has to have enough nucleophilicity to cleave the carbon-hetero atom bond and to open the cyclic monomer. Furthermore it is necessary that the build anion of the hetero-atom is nucleophilic enough to open further rings in order to achieve a successful polymerization. Most of the initiators are oxygen, sulfur or nitrogen anions with metallic countercations. For our synthesis we use an initiator system, shown in figure 1 of stannous octoate and figure 2 of octadecanol.
![]() Figure 1: structure of octadecanol
Figure 1: structure of octadecanol
![]() Figure 2: structure of Stannous Octoate
Figure 2: structure of Stannous Octoate
The mechanism of initiator systems out of stannous octoate [Sn(Oct)2] and alkohols [ROH] were severely discussed, but nowadays it is mostly accepted that the two components react to a stannous alkoxide Sn(OR)2 and octanoic acid OctH.
The stannous alkoxide is the initiating species for the anionic ring opening polymerization. The alkoxide species have a higher nucleophilicity than the octoat anion and can easily initiate reactions that octoate anions cannot initiate.
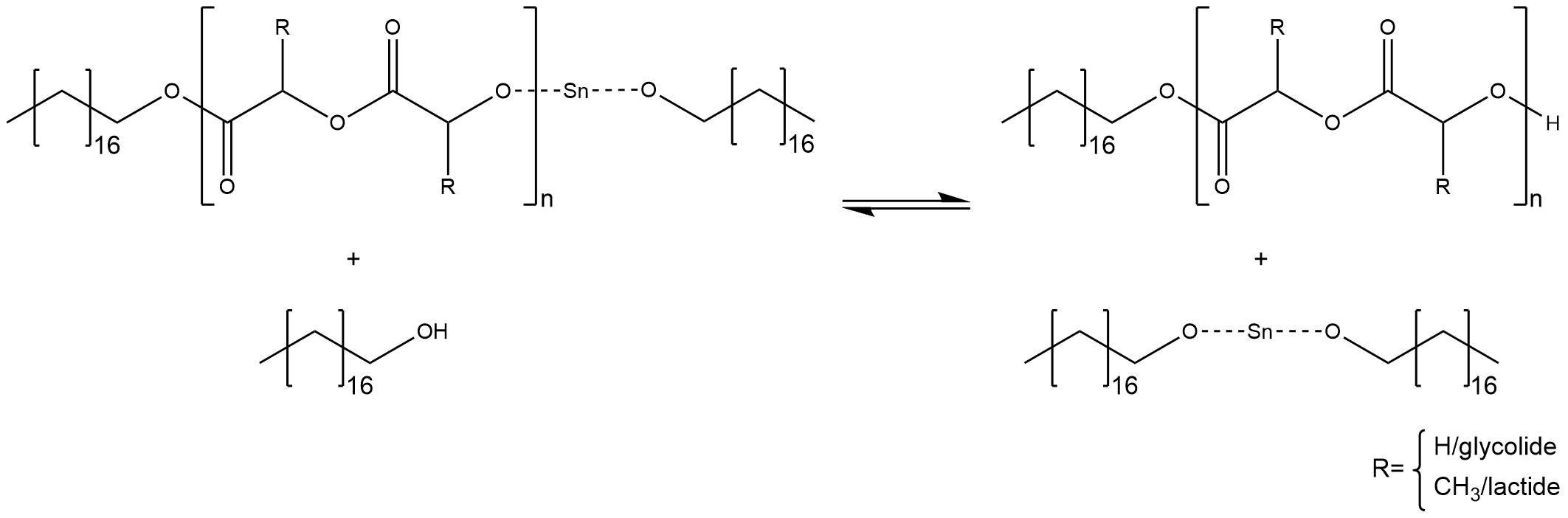
If the reactive species is built inside the reaction medium, the polymerization starts with a coordination insertion mechanism. That means one alcoholate ion of stannous alkoxide attacks the carbonylic carbon atom, while the tin cation coordinates the sp2-hybridized oxygen of the cyclic ester. Thus the ring opens at the sp3-hybridized oxygen and the build anion regenerates a new tin alkoxide, as shown in figure 4.
Figure 4
To sum up, one cyclic ester has been inserted. After the tin alkoxide is regenerated, the mechanism repeats and the chain grows.
The anionic ring opening polymerization can be realized by a polymerization out of a solution or out of a monomer melt. Given that lactide and glycolid are slightly soluble in common solvents, we decided to use the polymerization out of the monomer melt. Through a high vacuum and high temperatures, the moisture is eliminated out of our reaction vessel using Schlenk-technique. It is necessary to eliminate the moisture because water molecules deactivate the initiator molecules.
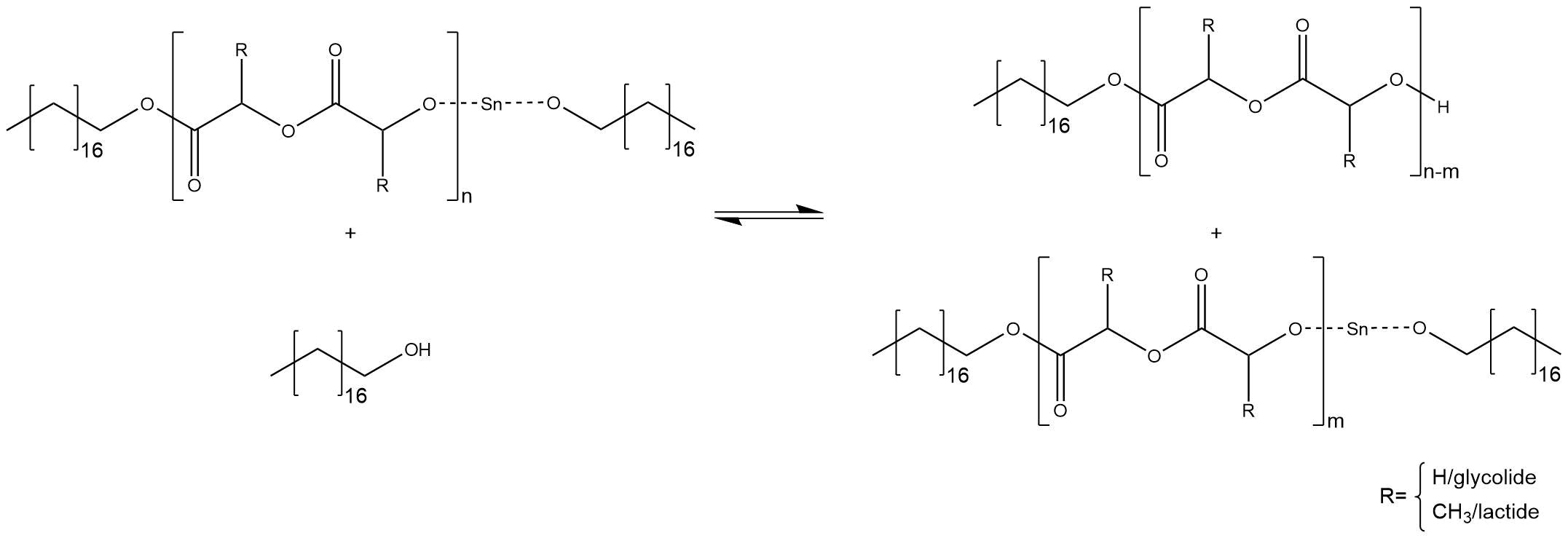
Controllable molecular weight depending on the ratio of initiator to monomers: One activated co-initiator starts one growing chain. If the stoichiometric ratio between initiator and co-initiator is right, there is no excess of the alcohol and all alcohol molecules are converted into the reactive stannous alkoxide. Statistically every single chain grows with the same velocity until the monomers are completely converted into polymer. Thus, it is possible to calculate the amount of monomers inside the polymer chains, which should have nearly the same size. An increase of the ratio between initiator to monomer causes smaller chains and, in reverse, a decrease of the ratio causes bigger molecular weights. Therefore, it is important to stress that a predictable molecular weight needs a conversion of 100%. If there is an excess of the co-initiator through using more co-initiator or less initiator, some free alcohol molecules causes side reactions. The alcohol attacks the tin-alkoxide at the end of a chain and substitute the proton of the hydroxyl group, regenerating the free active co-initiator, as shown in figure XY.
Figure 5
The chain cannot grow further, the co-initiator can start a new chain. Another side reaction is a substitution inside a chain, which breaks down the chain into two smaller chains, as shown in figure XY.
Figure 6
The chain, which bonds the tin alkoxide, can grow further. The main problem of these side reactions is the fact that chains with different sizes are synthesized. Therefore, the right ratio between initiator and co-initiator is essential for a predictable molecular weight.
Another important aspect of the synthesis is the fact that the glycolide monomers are more reactive than the lactide monomers. Glycolide has one methyl group less than lactide. Therefore it is easier to attack glycolide because the carbonyl carbon atom is less stericaly hindered. That can lead the glycolide to accumulate to blocks inside the polymer and the ratio of glycolide inside the polymer can be higher than the ratio of inserted monomers. To prevent such blocks, it is necessary to have a homogeneous reaction medium with strong stirring and conversion rates on nearly 100%. If PLGA has glycolide blocks inside, it influences the properties. The solubility of PLGA is worse, the cristallinity is higher and the polymer degrades faster. Thus, it is important to guarantee a random distribution of monomers to prevent monomer blocks in the polymer chains.
Two set ups of PLGA were processed during our time in the laboratory through an anionic ring-opening polymerization. For that the monomers are put in a water free reaction vessel, melted and reacted with the initiators. The method is described in the method book.
PLGA (I) with a ratio lactide/glycolide of 75%/25%
and
PLGA (II) with a ratio lactide/glycolide of 67%/33%
Degradation of PLGA
Earlier it was mentioned, that PLGA is a biodegradable plastic. The reason why PLGA is biodegradable, whereas many other plastics are not, is the special structure. Plastics without any reactive areas or bonds on its polymer chains, like saturated hydrocarbon polymers or worse polyfluorinated polymers are not biodegradable. These cannot degrade, because the water or enzymes of microorganisms do not have the possibility to cleave any bonds inside. Polyesters like PLGA have a backbone with ester groups in regular intervals. Each one of these ester bonds can be cleaved by water or by enzymes.
To stay for PLGA, the degradation speed mainly depends on the ratio of monomers and the form of the polymer. Water has to diffuse inside the polymer to reach the reactive ester bonds. Therefore, a porous surface with gaps eases the diffusion of water, which causes a swelling of the polymer. After swelling the polymer starts to degrade into oligomers. The mechanism of the degradation is an ester hydrolysis. The ratio of monomers is the property largely influencing the degradation. Lactide has two more methyl groups than glycolide. This has two consequences. First, the methyl groups are non-polar. Thus, they shield the polymer from water and water needs more time to penetrate the polymer structure. Accordingly, it applies the more lactide there is inside the polymer, the longer the time of degradation. The second reason is, that the carbonylic carbon atom of lactide is near by the methyl group. Therefore, water has less space to attack and the possibility of hydrolysis is smaller than in glycolide. The oligomers further degrade into glycolic acid and lactic acid. Microorganisms can convert lactic acid and glycolic acid to CO2 and H2O. The form of the polymer influences the degradation through the size of its surface. If the polymer has a huge surface, there is more space for water to diffuse inside the polymer. However, if the density of the polymer is high, the surface of the polymer is smaller in relation to the mass of the polymer and it becomes more difficult for water to penetrate.
Results and Discussion
GPC Results
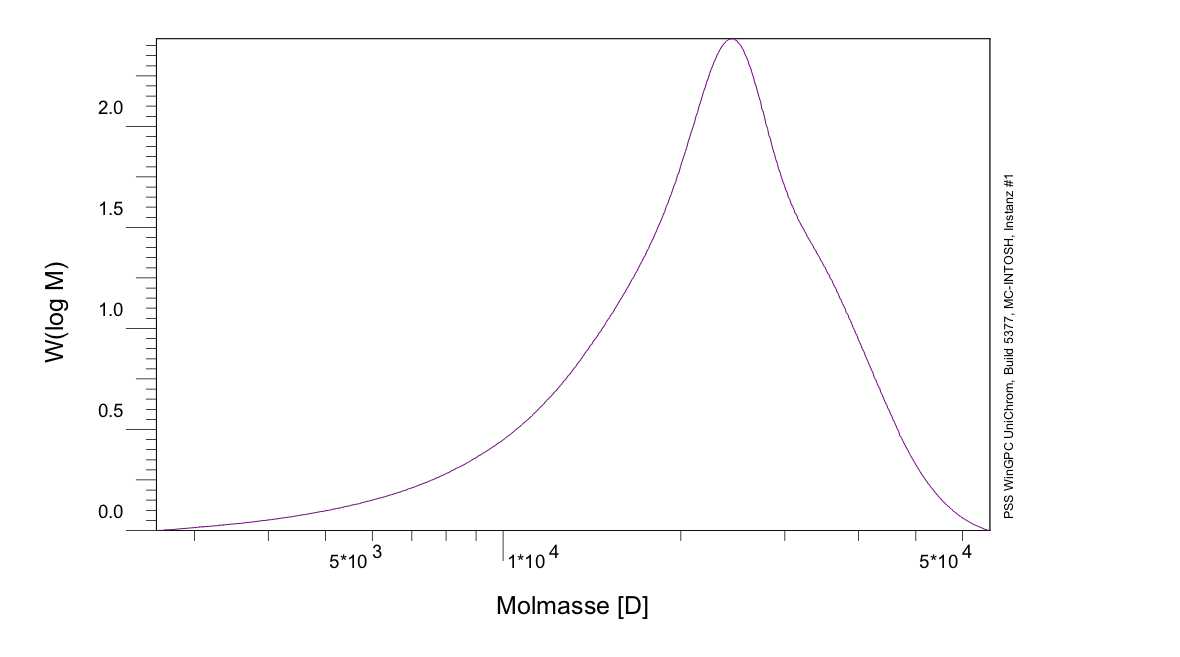
The expected molecular weight of PLGA (I) (ratio 75%/25%) was determined to be 361,669 g/mol, while the result of the GPC shows a molecular weight of 23,695 g/mol,as seen in figure 7, which means, that the chains are shorter than expected. Contrary to that the expected molecular weight of PLGA (II) (ratio 67%/33%) was determined to be 2.106*10^6 g/mol, while the GPC result shows a molecular weight of 167.9*10^6 g/mol, as seen in figure 8, which means, that in this case the chains are longer than expected. During an informative dialog with Evonik, they gave us the hint, that the magnetic stirring mechanism, we were using, is not sufficient enough to stir the reaction mix at higher viscosity. This leads to a affectation of the growth of the polymer chains, while the reaction mix solidified. To avoid this effect, more sufficient stirring mechanism is necessary.
Figure 7
GPC Graph von PLGA 2 Figure 8
Furthermore they gave us the hint that the molecular weight, which was higher than expected, results on a deactivation of initiator molecules by water, leading the monomers to spread on less polymer chains, increasing their length. This can be avoided by working in an even more water-freed environment.
This improvements however are not possible to be implememted in our laboratory set up, because, we are able to work either with sufficient stirring devices or in a water free environment.
NMR-Results
After the polymer was purified and dried, the yield of the synthesis was determined.
The maximal possible yield is approximately equal to the total mass of monomers: Table 1: used amount of monomers plus the yields of the synthesis (total and relative).
| Polymer | m (lactide) [g] | m (glycolide) [g] | Total Mass [g] | Total Yield [g] | Relative Yield [%] |
|---|---|---|---|---|---|
| PLGA (I) | 10.810 | 2.901 | 13.7 | 2.703 | 19,72 |
| PLGA (II) | 13.13 | 5.26 | 18.43 | 3.306 | 17.94 |
As the yields in table 1 shows, the relative yields are between[....]%. The yields are small. That is caused that we have not the right equipment to guaranty a homogeny melt. Thus with the progress of the synthesis, the viscosity of the melt becomes higher. During the synthesis the magnetic stirring becomes weaker. After a certain time, the stirring stops. At this moment we stop the reaction to have results we can compare. The time the reaction needs depends on the amount of monomers and the ratio of monomers to co-initiator. It would be necessary to use mechanical stirring to guaranty a homogeny melt to a conversion of 100%.
NMR-Spectroscopy:
To analyze the ratios inside our PLGA co-polymer we used 1H NMR-spectroscopy. We normalized the CH-group of lactide to 1. In this way we could determine the ratio of the monomers about equation 2:
To calculate the ratios, it is important to use the CH3-group of lactide and not the normalized Ch-group. This equations is valid for all monomer ratios.
Table 2: monomer ratio of PLGA polymers determined by NMR-spectroscopy.
| Polymer | Percentage of Lactide [%] | Percentage of Lactide [%] |
|---|---|---|
| PLGA (I) | 59.13 | 40.87 |
| PLGA (II) | 38.76 | 61.24 |
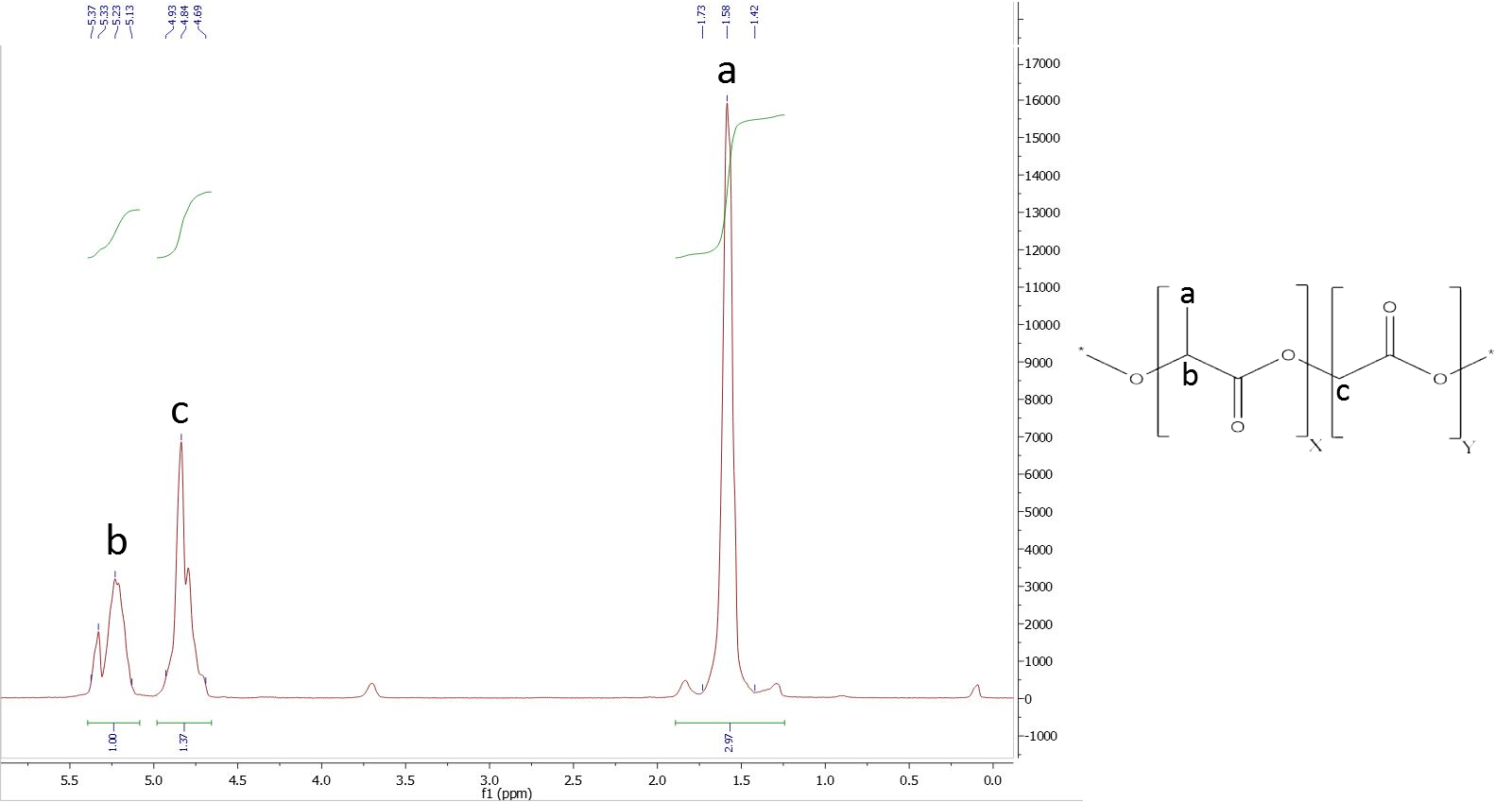
To determine the composition it is necessary to assign the peaks belonging to a specific proton group of a monomer. The figure 1 shows the structure of PLGA and its belonging NMR-spectra. The obtained areas of the peaks are typical for the PLGA co-polymer. The used peaks are the methyl protons of lactide with a shift of δ=1.44-1.69 plus the glycolide protons with a shift of δ=4.69-4.93 and the α methylene protons at a shift of δ=3.66-3.73.
Figure 9
Aus den NMR-Spektren geht hervor, dass das Verhältnis der Monomere im synthetisierten Polymer nicht den eingesetzten Verhältnissen entsprechen [Tab. 3]. Die Menge an eingebautem Glycolid ist höher. Damit bestätigen die Ergebnisse, dass Glycolid deutlich reaktiver ist als Lactid.
Table 3: theoretische und erhaltene Verhältnisse im Vergleich
| Polymer | Eingesetztes Monomerverhältnis [%] | Monomerverhältnis nach Synthese [%] |
|---|---|---|
| PLGA (I) | 75/25 | 59.13/40.87 |
| PLGA (II) | 66.6/33.3 | 38.76/61.24 |
Ist das anfänglich Verhältnis an Glycolid höher wirkt sich das deutlich auf das Verhältnis im Polymer aus. Wird die Menge an eingesetztem Glycolid von 25- auf 33% erhöht, dreht sich das resultierende Verhältnis (L/G) von 60/40 zu 40/60. Um trotz dieser unterschiedlichen Reaktivitäten die resultierenden Verhältnisse vorhersagen zu können, probieren wir anhand der Ergebnisse Geschwindigkeitskonstanten für das Einbauen eines der Monomere zu bestimmen. Mit Hilfe dieser soll anschließend ein kinetisches Modell erstellt werden, dass je nach eingesetztem Monomerverhältnis das entstehende Verhältnis im Polymer berechnet. Außerdem kann mit Hilfe des kinetischen Modells die Wahrscheinlichkeit von Glycolidblöcken bestimmt werden. Diese führen bei einer Synthese mit einem Ausgangsverhätnis von 1:1 zu einer veränderung der Löslichkeit. Das PLGA verändert das Löslichkeitsverhalten.(Weiter Infos zum modellieren finden Sie hier)
Outlook
PLGA and PLGC were produced using an insufficient magnetic stirring mechanism, which was not able to stir the reaction mixture at higher viscosity up to the end of the reaction. That led to a low conversion, which makes the results hardly reproducible. To avoid that, the reaction vessel needs to be designed in a way, which provides a continuous stirring all time throughout the reaction. The biggest difficulty lies in the achievement being able to have a mechanical stirring mechanism and pulling all humid atmospheric air out of the reaction vessel. Those conditions are not achievable with the laboratory equipment, which we can afford. With both conditions given, we would be able to get better reproducible results and more constant chain lengths. And with the polymers produced that way we would be also able to add additives and produce for example composite materials out of them.