Contents
PLGC
Abstract
Due to the fact, that PLGC is similar constructed to PLGA, the way of manufacturing is the same. PLGC is also synthesized via an anionic ring-opening polymerization. Both, the mechanism and the problems during the synthesis are identical. The essential difference lies in both the mechanical characteristics, which is explained in the background, and the degradation behaviors of both polymers. The degradation behavior is an important polymer characteristic for all applications of PLGA and PLGC. Therefore we will focus on the structural difference, which leads to different characteristics. To analyze the synthesized PLGC, we use similar to PLGA, GPC and NMR-spectroscopy. After that we used our purified polymer to manufacture PLGC nanospheres.
Introduction
PLGC is like PLGA, Polycarbonate (PC) and Polyethylenterephtalate (PET) also a thermoplastic polyester. The characteristics of PLGC are similar to those of PLGA, but it appears to be less stable, and degrades faster in aqueous environment. That leaves the advantage of PLGC as a drug delivery system, which releases therapeutics more controlled than systems made of PLGA.
Synthesis of PLGC
The synthesis of PLGC tri-co-polymers is equal to the synthesis of PLGA. Stannous-octoate and 1-octadecanol are forming the initiator part, reacting with lactide, glycolide and ε-caprolactone monomers to a polymer chain. Similar to the PLGA synthesis, the speed of the monomer conversion varies depending on the monomer itself. Glycolide reacts faster than lactide, while ε-caprolactone has the lowest conversion speed.
During our time in the laboratory two set ups of PLGC were processed with through an anionic ring-opening polymerization. For that the monomers were put in a water free reaction vessel, melted and reacted with the initiators. The method is described in detail in the method book.
PLGC (I) with a ratio lactid/glycolide/ε-caprolactone of 71%/19%/10%
and
PLGC (II) with a ratio lactid/glycolide/ε-caprolactone of 46%/44%/10%
Degradation of PLGC
Through the usage of ε-caprolactone, two competing effects occur. As described in the background, the PLGC mostly exists in its rubbery state, because of its low glass temperature (Tg). Due to that, the ε-caprolactone makes the polymer chain more flexible, allowing water molecules to diffuse better to the ester bonds in those chains, hydrolyzing them. Contrary to that, the ε-caprolactone lowers the polarity of the chains making them less hydrophilic. Due to that the water molecules are hindered to come near the ester bonds to hydrolyze them.
A small mole fraction of the ε-caprolactone in the chain, the degradation of PLGC proceeds faster than the degradation of PLGA. However, with the mole fraction of ε-caprolactone increasing, the polymer becomes more and more non-polar. Due to that, the degradation time increases compared to the degradation time of PLGA.
Results and Discussion
Analysis through GPC
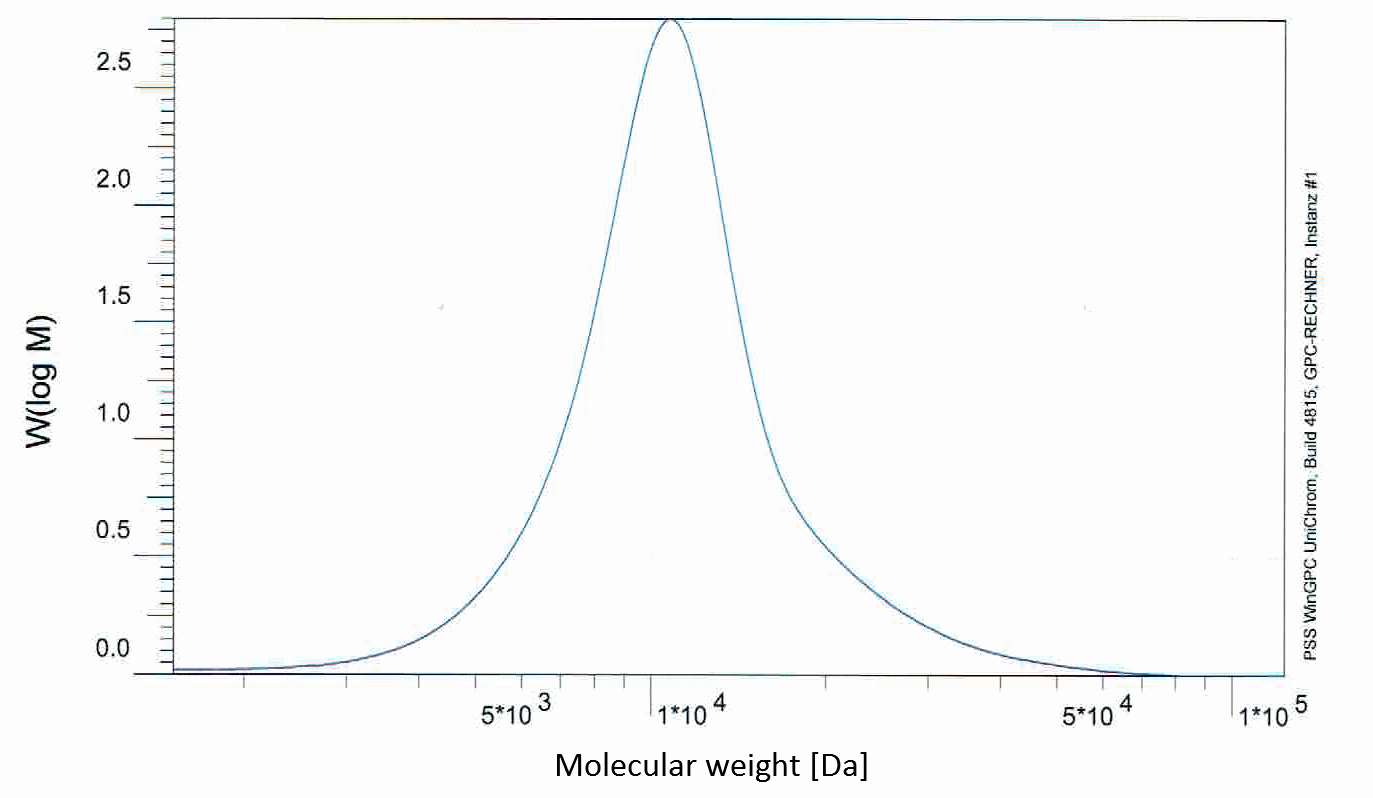
Graphen der GPCs
The expected molecule weight of PLGC (I) (ratio 71%/19%/10%) was determined to be 891,076 g/mol, while the GPC result shows a molecule weight of 12,224 g/mol, showing, that the polymer chains are shorter than expected. For the PLGC (II) (ratio 46%/44%/10%) the GPC result shows a molecule weight of 6,818 g/mol, while its expected molecule weight was determined to be 2.16*10^6 g/mol, which means that oligomers were produced instead of polymers.
Similar to the PLGA results the chains are shorter, because of the insufficient magnetic stirring device. The mixture is not stirred well enough at higher viscosity, so the growth of chains is affected while the mixture solidifies. And like in the case of PLGA this can be avoided by a more sufficient stirring mechanism.
But this effect does not completely explain, why the molecule weight of PLGC (II) is so low compared to the expected weight. The failure lies in the shutting down of the reaction and restarting it after a pause. The end of the chains seemed not to be reactivated, so the chain growth stopped during the pause. This leaves the conclusion, that an anionic polymerization can not be reactivated after it was shut down once.
The sufficient stirring device can not be implementated in our laboratory set up without losing the condition of a waterfree environment. Furthermore the reactions must not be shut down, before they are completed, to guarantee the production of polymer.
Analysis through NMR
Yields:
The calculation of yields for all synthesized PLGC polymers was performed identical as for PLGA polymers. Therefore, equation 1 from the PLGA page was used. Table 1 contains the resulting values.
Tabel 1: Used amounts of monomers and yields of synthesis (total and relative).
| Polymer | m (lactide) [g] | m (glycolide) [g] | m (ε-caprolactone) | Total Mass [g] | Total Yield [g] | Relative Yield [%] |
|---|---|---|---|---|---|---|
| PLGA (I) | 1.685 | 0.483 | 0.240 | 2.412 | 0.38 | 15.75 |
| PLGA (II) | 6.68 | 5.72 | 1.17 | 13.78 | 0.494 | 3.58 |
For PLGC (I) we purified 100 % of our product and calculated the yield as shown in table 1. Since PLGC (II) was further used for nanosphere synthesis, the actual total yield could not be determined. Therefore, the yield of PLGC (II) is a lot lower than for PLGC (I).
The added ε-caprolactone does not cause a change in viscosity in the reaction mixture. Since PLGC synthesis is the same as PLGA synthesis, the same problems occur. Yields tend to be low, as well.
NMR-spectroscopy
A 1H-NMR spectroscopy was used to determine the monomer ratio. The CH-group of lactide was normalized to 1. Through this, we gained integrals, which were inserted into equation 1 to calculate the monomer ratio. The ratios are shown in table 2. As signals of ε-caprolactone overlap with the ones from lactide and glycolide, a 2D-NMR spectrum was recorded. This allows a better differentiation between the individual protons of the molecules. The signals of α- and ϵ-methly groups can be distinguished clearly, since their proximity to the oxygen atoms deshields the ϵ-methly groups the strongest. Therefore, the ϵ-methylene signal shows a shift of δ =3.66-3.73 ppm. This signal is also used for the calculation of the monomer´s ratio. For lactide we used the methyl protons with a shift of δ=1.44-1.69 ppm, and for glycolide the methyl protons with a shift of δ=4.69-4.93 ppm. All peaks are shown in the following NMR-spectrum (Figure x).
Figure:
The spectrum of our synthesized PLGC is shown. All integrals are normalized to the CH-group of lactide. The important peaks are 1.44-1.69 ppm, illustrating the methyl protons of lactide, 3.66-3.73 ppm for the ε-caprolacton´s methyl group and 4.69-4.93 ppm for the glycolide methyl group. The ratio is calucated with the following equation:
The speed in which the monomers are incorporated into the polymer vary, as well. Generally, glycolide in inserted faster than lactide, while the incorporation of caprolactone is the slowest. Table 2 compares the relative monomer amounts used for synthesis with the relative amounts of incorporated monomers.
Tabel 2: Mole fractions of inserted monomers compared to the mole fractions in the produced polymer. The ratio of ε-caprolactone varies, even if it´s own concentration is initially identical, but the amounts of lactide and glycolide vary.
| Polymer | Inserted monomer ratio (L/G/C) [%] | Monomer ratio after synthesis (L/G/C) [%] |
|---|---|---|
| PLGA (I) | 71/19/10 | 47.6/47.6/4.8 |
| PLGA (II) | 46/44/10 | 46.2/51.2/2.6 |
ε-caprolactone varies, even if it´s own concentration is initially identical, but the amounts of lactide and glycolide vary.
The ratios gained through NMRspectroscopy show that the speed of ε-caprolactone´s incorporation is the slowest of all three monomers. In PLGC(II) the relative amount of ε-caprolactone monomer was decreased by 74 %, compared to the initial relative amount of free ε-caprolactone. As for PLGA synthesis, glycolide incorporation is again the fastest.
Outlook
PLGA and PLGC were produced using an insufficient magnetic stirring mechanism, which was not able to stir the reaction mixture at higher viscosity up to the end of the reaction. That led to a low conversion, which makes the results hardly reproducible. To avoid that, the reaction vessel needs to be designed in a way, which provides a continuous stirring all time throughout the reaction. The biggest difficulty lies in the achievement being able to have a mechanical stirring mechanism and pulling all humid atmospheric air out of the reaction vessel. Those conditions are not achievable with le laboratory equipment, which we can afford. With both conditions given, we would be able to get better reproducible results and more constant chain lengths. And with the polymers produced that way we would be also able to add additives and produce for example composite materials out of them.